PHF6 promotes the progression of endometrial carcinoma by increasing cancer cells growth and decreasing T-cell infiltration
- PMID: 36756714
- PMCID: PMC9983320
- DOI: 10.1111/jcmm.17638
PHF6 promotes the progression of endometrial carcinoma by increasing cancer cells growth and decreasing T-cell infiltration
Abstract
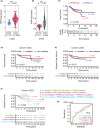
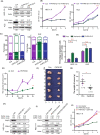
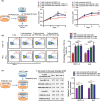
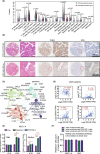
Uterine corpus endometrial carcinoma (UCEC) is the most common cancer of the female reproductive tract. The overall survival of advanced and recurrent UCEC patients is still unfavourable nowadays. It is urgent to find a predictive biomarker and block tumorgenesis at an early stage. Plant homeodomain finger protein 6 (PHF6) is a key player in epigenetic regulation, and its alterations lead to various diseases, including tumours. Here, we found that PHF6 expression was upregulated in UCEC tissues compared with normal tissues. The UCEC patients with high PHF6 expression had poor survival than UCEC patients with low PHF6 expression. PHF6 mutation occurred in 12% of UCEC patients, and PHF6 mutation predicted favourable clinical outcome in UCEC patients. Depletion of PHF6 effectively inhibited HEC-1-A and KLE cell proliferation in vitro and decreased HEC-1-A cell growth in vivo. Furthermore, high PHF6 level indicated a subtype of UCECs characterized by low immune infiltration, such as CD3+ T-cell infiltration. While knockdown of PHF6 in endometrial carcinoma cells increased T-cell migration by promoting IL32 production and secretion. Taken together, our findings suggested that PHF6 might play an oncogenic role in UCEC patients. Thus, PHF6 could be a potential biomarker in predicting the prognosis of UCEC patients. Depletion of PHF6 may be a novel therapeutic strategy for UCEC patients.
Keywords: PHF6; cell cycle; clinical outcome; epigenetic regulated gene; gene regulation; immune infiltration; uterine corpus endometrial carcinoma.
© 2022 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Najafi M, Majidpoor J, Toolee H, Mortezaee K. The current knowledge concerning solid cancer and therapy. J Biochem Mol Toxicol. 2021;35:e22900. - PubMed
-
- Burstein HJ, Krilov L, Aragon‐Ching JB, et al. Clinical cancer advances 2017: annual report on Progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2017;35:1341‐1367. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

