Prospective Validation of the Lémann Index in Children: A Report From the Multicentre Image Kids Study
- PMID: 36756849
- PMCID: PMC11004934
- DOI: 10.1093/ecco-jcc/jjad017
Prospective Validation of the Lémann Index in Children: A Report From the Multicentre Image Kids Study
Abstract
Background: The Lémann Index [LI] and the recently updated LI are tools for measuring structural bowel damage in adults with Crohn's disease [CD] but have not been evaluated in children. We aimed to validate the updated LI in the prospective multicentre ImageKids study of paediatric CD.
Methods: We included children with CD undergoing magnetic resonance enterography [MRE], pelvic magnetic resonance imaging [MRI] and ileocolonoscopy. Half were followed for 18 months, when MRE was repeated. Serum was collected for fibrosis-related proteomic markers. The LI was calculated by central readers from the MRE, ileocolonoscopy, physical examination and surgical data. Reliability and construct validity were assessed at baseline, while responsiveness and test-retest reliability were explored longitudinally.
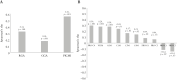
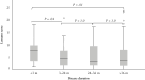
Results: In total, 240 children were included (mean age, 14.2 ± 2.5 years; median disease duration, 2.2 years [interquartile range, IQR 0.25-4.42]; median baseline LI, 4.23 [IQR 2.0-8.8]). The updated LI had excellent inter-observer reliability (interclass correlation coefficient [ICC] = 0.94, 95% confidence interval [CI] 0.92-0.95) but poor, although statistically significant, correlation with radiologist and gastroenterologist global assessments of damage and with serum proteomic levels of fibrotic markers [rho = 0.15-0.30, most p < 0.05]. The updated LI had low discriminative validity for detecting damage (area under the receiver operating characteristic curve [AUC-ROC] 0.69, 95% CI 0.62-0.75). In 116 repeated MREs, responsiveness was suboptimal for differentiating improved from unchanged disease [AUC-ROC 0.58, 95% CI 0.45-0.71]. Test-retest reliability was high among stable patients [ICC = 0.84, 95% CI 0.72-0.91].
Conclusion: Overall, the updated LI had insufficient psychometric performance for recommending its use in children. An age-specific index may be needed for children with shorter disease duration than typical adult cohorts.
Keywords: Crohn’s disease; Lemann score; MRE; Paediatrics.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
GF—received in the past 3 years consultancy fees from Abbvie and Lilly. RCK—received in the past 3 years consultancy fees from Alnylam. MLG—received in the past 3 years an AbbVie investigator-initiated research grant and honoraria, Samsung honoraria. LP, DAC, RNB, BY, AI, OK—None. PC—received in the past 3 years speaker fees, consultancy fees or grants from Abbvie, Amgen, Janssen, Takeda and Viatris. VMNL—received in past 3 years speaker fees, consultancy fees or grants from Abbvie, Rb, Nestle Health Science, Ordesa, Lactalis and Adacyte Therapeutics. JHM—None. FR—received in past 3 years consultancy fees from Adnovate, Agomab, Allergan, AbbVie, Arena, Boehringer-Ingelheim, Celgene/BMS, CDISC, Celsius, Cowen, Ferring, Galapagos, Galmed, Genentech, Gilead, Gossamer, Guidepoint, Helmsley, Horizon Therapeutics, Image Analysis Limited, Index Pharma, Jannsen, Koutif, Mestag, Metacrine, Mopac, Morphic, Organovo, Origo, Pfizer, Pliant, Prometheus Biosciences, Receptos, RedX, Roche, Samsung, Surmodics, Surrozen, Takeda, Techlab, Theravance, Thetis, UCB, Ysios and 89Bio. AMG—received in the past 3 years consultancy fees from Abbvie, Amgen, Bristol Myers Squibb, Lilly, Janssen, Merck, Pfizer, Takeda; speaker fees from Abbvie, Janssen; investigator-initiated research grant from Abbvie. DT—received in the past 3 years consultancy fees, research grant, royalties or honorarium from Janssen, Pfizer, Hospital for Sick Children, Ferring, Abbvie, Takeda, Atlantic Health, Shire, Celgene, Lilly, Roche, ThermoFisher, BMS.
Figures

References
-
- Levine A, Griffiths A, Markowitz J, et al. . Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis 2011;17:1314–21. - PubMed
-
- Henderson P, Hansen R, Cameron FL, et al. . Rising incidence of pediatric inflammatory bowel disease in Scotland. Inflamm Bowel Dis 2012;18:999–1005. - PubMed
-
- Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet 2017;389:1741–55. - PubMed
-
- Ng SC, Shi HY, Hamidi N, et al. . Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018;390:2769–78. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

