SARS-CoV-2 infection of kidney tissues from severe COVID-19 patients
- PMID: 36756942
- PMCID: PMC10388714
- DOI: 10.1002/jmv.28566
SARS-CoV-2 infection of kidney tissues from severe COVID-19 patients
Abstract
Background: Coronavirus disease 2019 (COVID-19) caused by infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) manifests diverse clinical pathologies involving multiple organs. While the respiratory tract is the primary SARS-CoV-2 target, acute kidney injury is common in COVID-19 patients, displaying as acute tubular necrosis (ATN) resulting from focal epithelial necrosis and eosinophilia, glomerulosclerosis, and autolysis of renal tubular cells. However, whether any renal cells are infected by SARS-CoV-2 and the mechanism involved in the COVID-19 kidney pathology remain unclear.
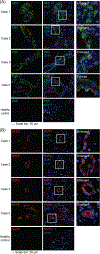
Methods: Kidney tissues obtained at autopsy from four severe COVID-19 patients and one healthy subject were examined by hematoxylin and eosin staining. Indirect immunofluorescent antibody assay was performed to detect SARS-CoV-2 spike protein S1 and nonstructural protein 8 (NSP8) together with markers of different kidney cell types and immune cells to identify the infected cells.
Results: Renal parenchyma showed tissue injury comprised of ATN and glomerulosclerosis. Positive staining of S1 protein was observed in renal parenchymal and tubular epithelial cells. Evidence of viral infection was also observed in innate monocytes/macrophages and NK cells. Positive staining of NSP8, which is essential for viral RNA synthesis and replication, was confirmed in renal parenchymal cells, indicating the presence of active viral replication in the kidney.
Conclusions: In fatal COVID-19 kidneys, there are SARS-CoV-2 infection, minimally infiltrated innate immune cells, and evidence of viral replication, which could contribute to tissue damage in the form of ATN and glomerulosclerosis.
Keywords: COVID-19; SARS-CoV-2; acute tubular necrosis, ATN; glomerulosclerosis; renal cell infection.
© 2023 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

