SAMD9 Promotes Postoperative Recurrence of Esophageal Squamous Cell Carcinoma by Stimulating MYH9-Mediated GSK3β/β-Catenin Signaling
- PMID: 36757050
- PMCID: PMC10104667
- DOI: 10.1002/advs.202203573
SAMD9 Promotes Postoperative Recurrence of Esophageal Squamous Cell Carcinoma by Stimulating MYH9-Mediated GSK3β/β-Catenin Signaling
Abstract
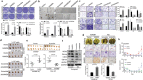
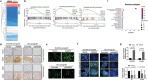
Recurrence is a challenge to survival after the initial treatment of esophageal squamous cell carcinoma (ESCC). But, its mechanism remains elusive and there are currently no biomarkers to predict postoperative recurrence. Here, the possibility of sterile alpha motif domain-containing protein 9 (SAMD9) as a predictor of postoperative recurrence of ESCC is evaluated and the molecular mechanisms by which SAMD9 promotes ESCC recurrence are elucidated. The authors found that the high level of SAMD9 is correlated with postoperative recurrence and poor prognosis of ESCC. Overexpression of SAMD9 promotes tumor stemness, angiogenesis, and EMT, while downregulation of SAMD9 reduced these phenotypes. Mechanistically, it is found that SAMD9 stimulated ubiquitination-mediated glycogen synthase kinase-3 beta (GSK-3β) degradation by interaction with myosin-9 (MYH9) and TNF receptor-associated factor 6 (TRAF6), which in turn activated Wnt/β-catenin pathway. Further, the authors demonstrated that silencing SAMD9 inhibited lung metastasis and tumor formation in vivo. Finally, the authors found that silencing MYH9 or β-catenin, or overexpressing GSK-3β inhibited SAMD9-stimulated ESCC cell stemness, EMT, angiogenesis, metastasis, and tumorigenicity. Together, the findings indicate that the SAMD9/MYH9/GSK3β/β-catenin axis promotes ESCC postoperative recurrence and that SAMD9 is a crucial target for ESCC therapy. Additionally, SAMD9 has the potential as a predictor of postoperative recurrence in ESCC.
Keywords: esophageal squamous cell carcinoma recurrence; myosin-9; sterile alpha motif domain-containing protein 9; β-catenin signaling.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A., CA–Cancer J Clin 2018, 68, 394. - PubMed
-
- Sun Z. G., Yu L., Yang F., Gao W., Wang Z., Zhu L. M., Pol. J. Pathol. 2016, 67, 384. - PubMed
-
- Ma Q., Yu T., Ren Y. Y., Gong T., Zhong D. S., Biochem. Biophys. Res. Commun. 2014, 454, 157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
