Azithromycin to Prevent Sepsis or Death in Women Planning a Vaginal Birth
- PMID: 36757318
- PMCID: PMC10627427
- DOI: 10.1056/NEJMoa2212111
Azithromycin to Prevent Sepsis or Death in Women Planning a Vaginal Birth
Abstract
Background: The use of azithromycin reduces maternal infection in women during unplanned cesarean delivery, but its effect on those with planned vaginal delivery is unknown. Data are needed on whether an intrapartum oral dose of azithromycin would reduce maternal and offspring sepsis or death.
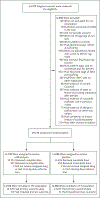
Methods: In this multicountry, placebo-controlled, randomized trial, we assigned women who were in labor at 28 weeks' gestation or more and who were planning a vaginal delivery to receive a single 2-g oral dose of azithromycin or placebo. The two primary outcomes were a composite of maternal sepsis or death and a composite of stillbirth or neonatal death or sepsis. During an interim analysis, the data and safety monitoring committee recommended stopping the trial for maternal benefit.
Results: A total of 29,278 women underwent randomization. The incidence of maternal sepsis or death was lower in the azithromycin group than in the placebo group (1.6% vs. 2.4%), with a relative risk of 0.67 (95% confidence interval [CI], 0.56 to 0.79; P<0.001), but the incidence of stillbirth or neonatal death or sepsis was similar (10.5% vs. 10.3%), with a relative risk of 1.02 (95% CI, 0.95 to 1.09; P = 0.56). The difference in the maternal primary outcome appeared to be driven mainly by the incidence of sepsis (1.5% in the azithromycin group and 2.3% in the placebo group), with a relative risk of 0.65 (95% CI, 0.55 to 0.77); the incidence of death from any cause was 0.1% in the two groups (relative risk, 1.23; 95% CI, 0.51 to 2.97). Neonatal sepsis occurred in 9.8% and 9.6% of the infants, respectively (relative risk, 1.03; 95% CI, 0.96 to 1.10). The incidence of stillbirth was 0.4% in the two groups (relative risk, 1.06; 95% CI, 0.74 to 1.53); neonatal death within 4 weeks after birth occurred in 1.5% in both groups (relative risk, 1.03; 95% CI, 0.86 to 1.24). Azithromycin was not associated with a higher incidence in adverse events.
Conclusions: Among women planning a vaginal delivery, a single oral dose of azithromycin resulted in a significantly lower risk of maternal sepsis or death than placebo but had little effect on newborn sepsis or death. (Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and others; A-PLUS ClinicalTrials.gov number, NCT03871491.).
Copyright © 2023 Massachusetts Medical Society.
Figures

Comment in
-
Excerpts from the World Medical Literature: Obstetrics.J Obstet Gynaecol Can. 2023 Jul;45(7):479-481. doi: 10.1016/j.jogc.2023.04.018. J Obstet Gynaecol Can. 2023. PMID: 37400184 No abstract available.
-
Azithromycin to Prevent Sepsis or Death in Women Planning a Vaginal Birth.N Engl J Med. 2023 Jul 20;389(3):282. doi: 10.1056/NEJMc2305875. N Engl J Med. 2023. PMID: 37467506 No abstract available.
-
Azithromycin to Prevent Sepsis or Death in Women Planning a Vaginal Birth.N Engl J Med. 2023 Jul 20;389(3):282-283. doi: 10.1056/NEJMc2305875. N Engl J Med. 2023. PMID: 37467507 No abstract available.
-
Azithromycin to Prevent Sepsis or Death in Women Planning a Vaginal Birth.N Engl J Med. 2023 Jul 20;389(3):283. doi: 10.1056/NEJMc2305875. N Engl J Med. 2023. PMID: 37467508 No abstract available.
-
Azithromycin to Prevent Sepsis or Death in Women Planning a Vaginal Birth. Reply.N Engl J Med. 2023 Jul 20;389(3):283-284. doi: 10.1056/NEJMc2305875. N Engl J Med. 2023. PMID: 37467509 Free PMC article. No abstract available.
References
-
- World Health Organization. WHO recommendations for the prevention and treatment of maternal peripartum infections September 28, 2015. (https://www.who.int/publications/i/item/9789241549363). - PubMed
-
- Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015;385:430–40. - PubMed
-
- reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing sepsis as a global health priority — a WHO resolution. N Engl J Med 2017; 377:414–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
