Antibiotics for chronic pulmonary infection in children with a neurodisability (neurodevelopmental disorder)
- PMID: 36757320
- PMCID: PMC9909774
- DOI: 10.1002/14651858.CD013813.pub2
Antibiotics for chronic pulmonary infection in children with a neurodisability (neurodevelopmental disorder)
Abstract
Background: 'Neurodisability' refers to a group of conditions that result primarily from a neurological problem (e.g. cerebral palsy), neuromuscular problem (e.g. a muscular dystrophy) or developmental problems (e.g. developmental impairment, Down syndrome). Children and young people with these conditions may have similar problems with mobility, feeding and airway clearance. Chest and breathing problems (including pulmonary infections) are commonly experienced by children and young people with neurodisabilities and are often a cause for them requiring hospital care. For those who are unable to completely clear their airway of secretions, or have frequent infections, pulmonary infections may not be able to be completely eradicated and therefore become chronic. It is unclear what treatment is best for children and young people in this position.
Objectives: To assess the effectiveness and adverse effects of antibiotic treatment for chronic pulmonary infection in children and young people living with a neurodisability, including quality-of-life measures, effects on hospitalisation and healthcare contacts.
Search methods: We searched the Cochrane Airways Trials Register, Cochrane Acute Respiratory Infections Group Register of Trials (CARIGRT), Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (Ovid), Embase (Ovid), Cumulative Index to Nursing and Allied Health Literature (CINAHL), OpenGrey (www.opengrey.eu) and three trials registries up to 8 February 2022. Additionally, we identified related systematic reviews through Epistemonikos.org (8 February 2022) and searched reference lists of these.
Selection criteria: All randomised controlled trials of antibiotic therapy for chronic pulmonary infection in children and young people up to the age of 18 living with a neurodisability were eligible.
Data collection and analysis: Two independent review authors screened results of the searches against predetermined inclusion criteria, resolving any discrepancies by discussion.
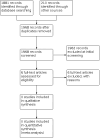
Main results: We identified a total of 1968 independent records through our searches, of which we assessed six full-text articles for eligibility. We identified one ongoing study as well as one related substudy but did not identify any completed studies eligible for inclusion in this systematic review.
Authors' conclusions: The findings of this systematic review highlight a lack of evidence in the antibiotic treatment of chronic pulmonary infection in children and young people up to the age of 18 living with a neurodisability. Further research examining this topic is therefore required.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
JS: salary part‐funded by NIHR Academic Clinical Fellowship.
KJ: none known.
JW: none known.
MNH: site Principal Investigator for the PARROT trial.
Update of
References
References to studies excluded from this review
Gleason 2007 {published data only}
-
- Gleason PP, Shaughnessy AF. Telithromycin (Ketek) for treatment of community-acquired pneumonia. American Family Physician 2007;76(12):1857-8.
Greenberg 1974 {published data only}
Nydahl‐Persson 1995 {published data only}
References to ongoing studies
ACTRN12621001486819 {published data only}
-
- ACTRN12621001486819. Effect of prophylactic azithromycin on chest infections in children with neurological and neuromuscular impairment [Randomised, triple blinded, placebo trial comparing 26 weeks of azithromycin to placebo in children with neurological/neuromuscular impairment to reduce the risk of lower respiratory tract infection]. trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12621001486819 (first received 1 November 2021). [ACTRN: ACTRN12621001486819]
ISRCTN71955516 {published data only}ACTRN12619001597189ISRCTN71955516
-
- ISRCTN71955516. Prophylactic antibiotics to prevent chest infections in children with neurological impairment [Joint UK and Australia multicentre, randomised, double-blind, placebo-controlled pragmatic trial comparing 52 weeks of azithromycin to placebo in children with neurological impairment at risk of lower respiratory tract infection (the PARROT trial)]. trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN71955516 (first received 22 April 2020). [DOI: ]
Additional references
Baars 2005
-
- Baars RM, Atherton C, Koopman HM, Bullinger M, Power M, DISABKIDS group. The European DISABKIDS project: development of seven condition-specific modules to measure health related quality of life in children and adolescents. Health and Quality of Life Outcomes 2005;3:70. [DOI: 10.1186/1477-7525-3-70] - DOI - PMC - PubMed
Blackmore 2019
-
- Blackmore AM, Gibson N, Cooper MS, Langdon K, Moshovis L, Wilson AC. Interventions for management of respiratory disease in young people with cerebral palsy: a systematic review. Child: Care, Health and Development 2019;45(5):754-71. - PubMed
Boel 2019
-
- Boel L, Pernet K, Toussaint M, Ides K, Leemans G, Haan J, et al. Respiratory morbidity in children with cerebral palsy: an overview. Developmental Medicine and Child Neurology 2019;61(6):646-53. - PubMed
Cassini 2019
-
- Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infectious Diseases 2019;19(1):56-66. - PMC - PubMed
Chang 2015
Cochrane Airways 2019
-
- Cochrane Airways Trials Register. airways.cochrane.org/trials-register (accessed 7 May 2019).
Covidence [Computer program]
-
- Covidence. Version accessed 25 October 2019. Melbourne, Australia: Veritas Health Innovation. Available at www.covidence.org.
Cunha 2001
-
- Cuhna BA. Antibiotic side effects. Medical Clinics of North America 2001;85:149-85. - PubMed
Davis 2013
-
- Davis E, Mackinnon A, Davern M, Boyd R, Bohanna I, Waters E, et al. Description and psychometric properties of the CP QOL-Teen: A quality of life questionnaire for adolescents with cerebral palsy. Research in Developmental Disabilities 2013;34(1):344-52. [DOI: 10.1016/j.ridd.2012.08.018] - DOI - PubMed
Dohna‐Schwake 2006
Elbourne 2002
Emerson 2012
Family Resources Survey 2016
-
- Department for Work and Pensions, UK. Family resources survey: financial year 2016/17. www.gov.uk/government/statistics/family-resources-survey-financial-year-... (accessed prior to 29 November 2020).
Fitzgerald 2009
-
- Fitzgerald DA, Follett J, Van Asperen PP. Assessing and managing lung disease and sleep disordered breathing in children with cerebral palsy. Paediatric Respiratory Reviews 2009;10(1):18-24. - PubMed
Gleckman 2000
-
- Gleckman RA, Czachor JS. Antibiotic side effects. Seminars in Respiratory and Critical Care Medicine 2000;21(1):61-70. - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 25 October 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook/archive/v5.2.
Higgins 2020
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook. - PMC - PubMed
Hum 2019
Hurley 2015
-
- Hurley M, Vyas H. Respiratory problems in children with neurodisability. Paediatrics and Child Health 2015;25(10):463-6.
Kansra 2016
-
- Kansra S, Ugonna K. Fifteen-minute consultation: approach to management of respiratory problems in children with neurodisability. Archives of Disease in Childhood Education and Practice Edition 2016;101(5):226-31. - PubMed
Kohanski 2010
Marshall 2018
McDonald 2017
-
- McDonald S, Noel-Storr AH, Thomas J. Harnessing the efficiencies of machine learning and Cochrane Crowd to identify randomised trials for individual Cochrane reviews. In: Global Evidence Summit; 2017 September 13-16; Cape Town, South Africa. 2017.
McGowan 2016
Moher 2009
Morris 2013
NCEPOD 2018
-
- National Confidential Enquiry into Patient Outcome and Death. Each and every need - a review of the quality of care provided to patients aged 0-25 years old with chronic neurodisability, using the cerebral palsies as examples of chronic neurodisabling conditions; 2018. www.ncepod.org.uk/2018report1/downloads/EachAndEveryNeed_FullReport.pdf.
Noel‐Storr 2018
-
- Noel-Storr AH, Project Transform team. Cochrane Crowd: new ways of working together to produce health evidence. In: Evidence Live; 2018 June 18-20; Oxford, UK. 2018.
Onakpoya 2015
Petrou 2013
Plioplys 2011
Proesmans 2016
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Thomas 2017
-
- Thomas J, Noel-Storr AH, Marshall I, Wallace B, McDonald S, Mavergames C, et al. Living systematic review network. Living systematic reviews: 2. Combining human and machine effort. Journal of Clinical Epidemiology 2017;91:31-7. - PubMed
Trinick 2015
-
- Trinick RE, Bunni L, Thorburn K, Hackett AP, Dalzell M, McNamara PS. An observational study examining the relationship between respiratory symptoms, airway inflammation and bacteriology in children with severe neurodisability. PLOS one 2015;10(4):e0124627. [DOI: 10.1371/journal.pone.0124627] - DOI - PMC - PubMed
References to other published versions of this review
Sanner 2020
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical