Robotic stereotactic body radiotherapy for localized prostate cancer: final analysis of the German HYPOSTAT trial
- PMID: 36757424
- PMCID: PMC10212861
- DOI: 10.1007/s00066-023-02044-2
Robotic stereotactic body radiotherapy for localized prostate cancer: final analysis of the German HYPOSTAT trial
Abstract
Purpose: We report results of the first German prospective multicenter single-arm phase II trial (ARO 2013-06; NCT02635256) of hypofractionated robotic stereotactic body radiotherapy (SBRT) for patients with localized prostate cancer (HYPOSTAT).
Methods: Patients eligible for the HYPOSTAT study had localized prostate cancer (cT1‑3 cN0 cM0), Gleason score ≤ 7, prostate-specific antigen (PSA) ≤ 15 ng/ml, prostate volume ≤ 80 cm3, and an International Prostate Symptom Score (IPSS) ≤ 12. Initially, inclusion was limited to patients ≥ 75 years or patients 70-74 years with additional risk factors. The trial protocol was later amended to allow for enrolment of patients aged ≥ 60 years. The treatment consisted of 35 Gy delivered in 5 fractions to the prostate and for intermediate- or high-risk patients, also to the proximal seminal vesicles using the CyberKnife system (Accuray Inc., Sunnyvale, CA, USA). Primary endpoint was the rate of treatment-related gastrointestinal or genitourinary grade ≥ 2 toxicity based on the RTOG scale 12-15 months after treatment. Secondary endpoints were acute toxicity, late toxicity, urinary function, quality of life, and PSA response.
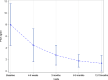
Results: From July 2016 through December 2018, 85 eligible patients were enrolled and received treatment, of whom 83 could be evaluated regarding the primary endpoint. Patients mostly had intermediate-risk disease with a median PSA value of 7.97 ng/ml and Gleason score of 7a and 7b in 43.5% and 25.9% of patients, respectively. At the final follow-up 12-15 months after treatment, no patient suffered from treatment-related gastrointestinal or genitourinary grade ≥ 2 toxicity. Acute toxicity was mostly mild, with three grade 3 events, and the cumulative rate of grade ≥ 2 genitourinary toxicity was 8.4% (95% CI 4.1-16.4%). There were no major changes in urinary function or quality of life. The median PSA value dropped to 1.18 ng/ml 12-15 months after treatment. There was one patient who developed distant metastases.
Conclusion: Robotic SBRT with 35 Gy in 5 fractions was associated with a favorable short-term toxicity profile. Recruitment for the HYPOSTAT‑2 trial (ARO-2018‑4; NCT03795337), which further analyses the late toxicity of this regimen with a planned sample size of 500 patients, is ongoing.
Keywords: Biochemical recurrence; CyberKnife; Hypofractionation; Quality of life; Radiation Oncology; Toxicity.
© 2023. The Author(s).
Conflict of interest statement
D. Krug has received honoraria from MSD Sharp & Dohme and Pfizer as well as research funding from Merck KGaA, outside the submitted work. A. Muacevic and C. Fürweger previously received speaker fees from Accuray. O. Blanck is the section editor for medical physics of the journal
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

