Cost-effectiveness of IVF with PGT-M/A to prevent transmission of spinal muscular atrophy in offspring of carrier couples
- PMID: 36757555
- PMCID: PMC10224878
- DOI: 10.1007/s10815-023-02738-7
Cost-effectiveness of IVF with PGT-M/A to prevent transmission of spinal muscular atrophy in offspring of carrier couples
Abstract
Purpose: To evaluate the cost-effectiveness of in-vitro fertilization with preimplantation genetic testing for aneuploidy and monogenic disorders (IVF with PGT-M/A) to prevent transmission of spinal muscular atrophy to offspring of carrier couples.
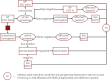
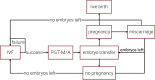
Methods: A decision-analytic model was created to compare the cost-effectiveness of IVF with PGT-M/A to unassisted conception with prenatal diagnostic testing and termination (if applicable). IVF with PGT-M/A costs were determined using a separate Markov state-transition model. IVF outcomes data was derived from 76 carriers of monogenic disorders who underwent IVF with PGT-M/A at a single academic REI center. Other probabilities, costs, and utilities were derived from the literature. Costs were modeled from healthcare perspective. Utilities were modeled from the parental perspective as quality-adjusted life-years (QALYs).
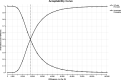
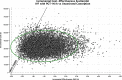
Results: The incremental cost-effectiveness ratio for IVF with PGT-M/A compared to unassisted conception is $22,050 per quality-adjusted life-year. The average cost of IVF with PGT-M/A is $41,002 (SD: $8,355). At willingness-to-pay thresholds of $50,000 and $100,000, IVF with PGT-M/A is cost-effective 93.3% and 99.5% of the time, respectively.
Conclusions: Compared to unassisted conception, IVF with PGT-M/A is cost-effective for preventing the transmission of spinal muscular atrophy to the offspring of carrier couples. These findings support insurance coverage of IVF with PGT-M/A for carriers of spinal muscular atrophy.
Keywords: Cost-effectiveness; IVF; PGTA; PGTM; Spinal muscular atrophy.
© 2023. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Figures
Similar articles
-
Cost effectiveness of in vitro fertilisation and preimplantation genetic testing to prevent transmission of BRCA1/2 mutations.Hum Reprod. 2020 Feb 29;35(2):434-445. doi: 10.1093/humrep/dez203. Hum Reprod. 2020. PMID: 32099994
-
Clinical validity and utility of preconception expanded carrier screening for the management of reproductive genetic risk in IVF and general population.Hum Reprod. 2021 Jun 18;36(7):2050-2061. doi: 10.1093/humrep/deab087. Hum Reprod. 2021. PMID: 34021342
-
Cost-effectiveness of preimplantation genetic testing for aneuploidy for women with subfertility in China: an economic evaluation using evidence from the CESE-PGS trial.BMC Pregnancy Childbirth. 2023 Apr 14;23(1):254. doi: 10.1186/s12884-023-05563-z. BMC Pregnancy Childbirth. 2023. PMID: 37060068 Free PMC article. Clinical Trial.
-
Preimplantation genetic testing for aneuploidies (abnormal number of chromosomes) in in vitro fertilisation.Cochrane Database Syst Rev. 2020 Sep 8;9(9):CD005291. doi: 10.1002/14651858.CD005291.pub3. Cochrane Database Syst Rev. 2020. PMID: 32898291 Free PMC article.
-
A Review of Cost-Effectiveness of Preimplantation Genetic Testing for Aneuploidy.Obstet Gynecol Surv. 2025 Mar;80(3):169-173. doi: 10.1097/OGX.0000000000001373. Obstet Gynecol Surv. 2025. PMID: 40080891 Review.
Cited by
-
Preimplantation genetic testing for sickle cell disease: a cost-effectiveness analysis.F S Rep. 2023 Jun 13;4(3):300-307. doi: 10.1016/j.xfre.2023.06.001. eCollection 2023 Sep. F S Rep. 2023. PMID: 37719105 Free PMC article.
-
Male Reproduction in Spinal Muscular Atrophy (SMA) and the Potential Impact of Oral Survival of Motor Neuron 2 (SMN2) Pre-mRNA Splicing Modifiers.Neurol Ther. 2024 Aug;13(4):933-947. doi: 10.1007/s40120-024-00626-5. Epub 2024 May 16. Neurol Ther. 2024. PMID: 38750391 Free PMC article.
-
Fertility preservation and assisted reproductive strategies in endometrial cancer patients with lynch syndrome.Front Oncol. 2025 Jul 21;15:1630301. doi: 10.3389/fonc.2025.1630301. eCollection 2025. Front Oncol. 2025. PMID: 40761251 Free PMC article. Review.
References
-
- BT D. Spinal muscular atrophies. Pediatr Clin North Am [Internet]. Pediatr Clin North Am; 2015 [cited 2021 Aug 17];62:743–66. Available from: https://pubmed-ncbi-nlm-nih-gov.laneproxy.stanford.edu/26022173/ - PubMed
-
- Droege M, Sproule D, Arjunji R, Gauthier-Loiselle M, Cloutier M, Dabbous O. Economic burden of spinal muscular atrophy in the United States: a contemporary assessment. J Med Econ [Internet]. Taylor and Francis Ltd; 2020 [cited 2020 Nov 19];23:70–9 10.1080/13696998.2019.1646263 - PubMed
-
- Landfeldt E, Edström J, Sejersen T, Tulinius M, Lochmüller H, Kirschner J. Quality of life of patients with spinal muscular atrophy: a systematic review. Eur J Paediatr Neurol. W.B. Saunders Ltd; 2019;23:347–56. - PubMed
-
- Prior TW, Leach ME, Finanger E. Spinal Muscular Atrophy. GeneReviews® [Internet]. University of Washington, Seattle; 2020 [cited 2021 Aug 17]; Available from: https://www-ncbi-nlm-nih-gov.laneproxy.stanford.edu/books/NBK1352/ - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

