Allogeneic Hematopoietic Cell Transplantation vs Standard Consolidation Chemotherapy in Patients With Intermediate-Risk Acute Myeloid Leukemia: A Randomized Clinical Trial
- PMID: 36757706
- PMCID: PMC9912165
- DOI: 10.1001/jamaoncol.2022.7605
Allogeneic Hematopoietic Cell Transplantation vs Standard Consolidation Chemotherapy in Patients With Intermediate-Risk Acute Myeloid Leukemia: A Randomized Clinical Trial
Abstract
Importance: The ideal postremission strategy in intermediate-risk acute myeloid leukemia (AML) in first complete remission (CR) has been a matter of debate.
Objective: To explore the optimal therapy for patients with intermediate-risk AML after first complete remission.
Design, settings, and participants: This investigator-initiated, open-label, 2-armed, phase 3 randomized clinical trial assessed patients at 16 hospitals in Germany from February 2, 2011, until July 1, 2018. Key eligibility criteria included cytogenetically defined intermediate-risk AML according to Medical Research Council classification, first CR or CR with incomplete blood cell count recovery after conventional induction therapy, age of 18 to 60 years, and availability of a human leukocyte antigen (HLA)-matched sibling or unrelated donor. A detailed statistical analysis plan was written and finalized on July 7, 2020. Data were exported for analysis on April 13, 2021.
Interventions: Patients were randomized 1:1 to receive allogeneic hematopoietic cell transplantation (HCT) or high-dose cytarabine for consolidation and salvage HCT only in case of relapse. Strata for randomization included age (18-40 vs 41-60 years), NPM1 and CEBPA variation status, and donor type (unrelated vs related).
Main outcomes and measures: End points included overall-survival as the primary outcome and disease-free survival, cumulative incidence of relapse, treatment-related mortality, and quality of life measured according to the Medical Outcomes Study 36-Item Short-Form Health Survey as secondary outcomes.
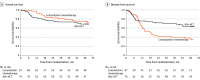
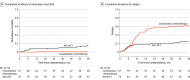
Results: A total of 143 patients (mean [SD] age, 48.2 [9.8] years; 81 [57%] male) with AML who fulfilled the eligibility criteria were randomized. In the intention-to-treat analysis, the probability of survival at 2 years was 74% (95% CI, 62%-83%) after primary allogeneic HCT and 84% (95% CI, 73%-92%) after consolidation chemotherapy (P = .22). Disease-free survival after HCT at 2 years was 69% (95% CI, 57%-80%) compared with 40% (95% CI, 28%-53%) after consolidation chemotherapy (P = .001). Allogeneic HCT during the first CR was associated with a cumulative incidence of relapse at 2 years of 20% (95% CI, 13%-31%) compared with 58% (95% CI, 47%-71%; P < .001). Nonrelapse mortality at 2 years after primary allogeneic HCT was 9% (95% CI, 5%-19%) and 2% (95% CI, 0%-11%) after consolidation chemotherapy (P = .005). Similar outcomes were observed when analyses were confined to the 96 patients at intermediate risk according to the European Leukemia Network classification. Most importantly, all 41 patients relapsing after consolidation chemotherapy (36 hematologic, 4 molecular, and 1 extramedullary) proceeded to allogeneic HCT. No significant differences in health-related quality of life measures were observed between groups.
Conclusions and relevance: Primary allogeneic HCT during first CR was not associated with superior overall survival compared with consolidation chemotherapy in patients 60 years or younger with intermediate-risk AML during the first CR and an available donor.
Trial registration: ClinicalTrials.gov Identifier: NCT01246752.
Conflict of interest statement
Figures

References
-
- Cancer Stat Facts . Leukemia—acute myeloid leukemia (AML). Accessed January 15, 2022. https://seer.cancer.gov/statfacts/html/amyl.html
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

