The clinical and genetic spectrum of autosomal-recessive TOR1A-related disorders
- PMID: 36757831
- PMCID: PMC10393417
- DOI: 10.1093/brain/awad039
The clinical and genetic spectrum of autosomal-recessive TOR1A-related disorders
Abstract
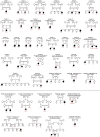
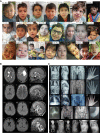
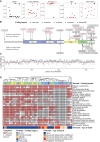
In the field of rare diseases, progress in molecular diagnostics led to the recognition that variants linked to autosomal-dominant neurodegenerative diseases of later onset can, in the context of biallelic inheritance, cause devastating neurodevelopmental disorders and infantile or childhood-onset neurodegeneration. TOR1A-associated arthrogryposis multiplex congenita 5 (AMC5) is a rare neurodevelopmental disorder arising from biallelic variants in TOR1A, a gene that in the heterozygous state is associated with torsion dystonia-1 (DYT1 or DYT-TOR1A), an early-onset dystonia with reduced penetrance. While 15 individuals with AMC5-TOR1A have been reported (less than 10 in detail), a systematic investigation of the full disease-associated spectrum has not been conducted. Here, we assess the clinical, radiological and molecular characteristics of 57 individuals from 40 families with biallelic variants in TOR1A. Median age at last follow-up was 3 years (0-24 years). Most individuals presented with severe congenital flexion contractures (95%) and variable developmental delay (79%). Motor symptoms were reported in 79% and included lower limb spasticity and pyramidal signs, as well as gait disturbances. Facial dysmorphism was an integral part of the phenotype, with key features being a broad/full nasal tip, narrowing of the forehead and full cheeks. Analysis of disease-associated manifestations delineated a phenotypic spectrum ranging from normal cognition and mild gait disturbance to congenital arthrogryposis, global developmental delay, intellectual disability, absent speech and inability to walk. In a subset, the presentation was consistent with foetal akinesia deformation sequence with severe intrauterine abnormalities. Survival was 71%, with higher mortality in males. Death occurred at a median age of 1.2 months (1 week-9 years), due to respiratory failure, cardiac arrest or sepsis. Analysis of brain MRI studies identified non-specific neuroimaging features, including a hypoplastic corpus callosum (72%), foci of signal abnormality in the subcortical and periventricular white matter (55%), diffuse white matter volume loss (45%), mega cisterna magna (36%) and arachnoid cysts (27%). The molecular spectrum included 22 distinct variants, defining a mutational hotspot in the C-terminal domain of the Torsin-1A protein. Genotype-phenotype analysis revealed an association of missense variants in the 3-helix bundle domain to an attenuated phenotype, while missense variants near the Walker A/B motif as well as biallelic truncating variants were linked to early death. In summary, this systematic cross-sectional analysis of a large cohort of individuals with biallelic TOR1A variants across a wide age-range delineates the clinical and genetic spectrum of TOR1A-related autosomal-recessive disease and highlights potential predictors for disease severity and survival.
Keywords: AMC5; NDD; Torsin-1A; arthrogryposis multiplex congenita 5; biallelic variation.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
M.M.M., I.M.W, L.B.H. and F.M. are employees of GeneDx, LLC. J.R.L. has stock ownership in 23andMe, is a paid consultant for Regeneron Genetics Center, and is a co-inventor on multiple US and European patents related to molecular diagnostics for inherited neuropathies, eye diseases, genomic disorders and bacterial genomic fingerprinting. The Department of Molecular and Human Genetics at Baylor College of Medicine receives revenue from clinical genetic and genomic testing conducted at Baylor Genetics (BG); J.R.L. serves on the Scientific Advisory Board (SAB) of BG. W.K.C. is on the scientific advisory board of the Regeneron Genetics Center and the Board of Directors of Prime Medicine. L.C. has served as a paid consultant for Horizon Therapeutics and PTC Therapeutics. M.T. has served as Ontario Ministry of Health Exceptional Access Program member for genetic testing approvals. N.G.-O. is a consultant and has equity interest in Graphite Bio and Codexis. All other authors declare no conflict of interest.
Figures

References
-
- Granata A, Watson R, Collinson LM, Schiavo G, Warner TT. The dystonia-associated protein torsinA modulates synaptic vesicle recycling. J Biol Chem. 2008;283:7568–7579. - PubMed
-
- Hewett JW, Zeng J, Niland BP, Bragg DC, Breakefield XO. Dystonia-causing mutant torsinA inhibits cell adhesion and neurite extension through interference with cytoskeletal dynamics. Neurobiol Dis. 2006;22:98–111. - PubMed
-
- Lange LM, Junker J, Loens S, et al. Genotype-phenotype relations for isolated dystonia genes: MDSGene systematic review. Mov Disord. 2021;36:1086–1103. - PubMed

