Two-year outcomes of leadless vs. transvenous single-chamber ventricular pacemaker in high-risk subgroups
- PMID: 36757859
- PMCID: PMC10062361
- DOI: 10.1093/europace/euad016
Two-year outcomes of leadless vs. transvenous single-chamber ventricular pacemaker in high-risk subgroups
Abstract
Aims: This study compares clinical outcomes between leadless pacemakers (leadless-VVI) and transvenous ventricular pacemakers (transvenous ventricular permanent-VVI) in subgroups of patients at higher risk of pacemaker complications.
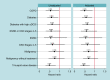
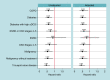
Methods and results: This study is based on the Micra Coverage with Evidence Development (CED) study. Patients from the Micra CED study were considered in a high-risk subgroup if they had a diagnosis of chronic kidney disease Stages 4-5 (CKD45), end-stage renal disease, malignancy, diabetes, tricuspid valve disease (TVD), or chronic obstructive pulmonary disease (COPD) 12 months prior to pacemaker implant. A pre-specified set of complications and reinterventions were identified using diagnosis and procedure codes. Competing risks models were used to compare reinterventions and complications between leadless-VVI and transvenous-VVI patients within each subgroup; results were adjusted for multiple comparisons. A post hoc comparison of a composite outcome of reinterventions and device complications was conducted. Out of 27 991 patients, 9858 leadless-VVI and 12 157 transvenous-VVI patients have at least one high-risk comorbidity. Compared to transvenous-VVI patients, leadless-VVI patients in four subgroups [malignancy, HR 0.68 (0.48-0.95); diabetes, HR 0.69 (0.53-0.89); TVD, HR 0.60 (0.44-0.82); COPD, HR 0.73 (0.55-0.98)] had fewer complications, in three subgroups [diabetes, HR 0.58 (0.37-0.89); TVD, HR 0.46 (0.28-0.76); COPD, HR 0.51 (0.29-0.90)) had fewer reinterventions, and in four subgroups (malignancy, HR 0.52 (0.32-0.83); diabetes, HR 0.52 (0.35-0.77); TVD, HR 0.44 (0.28-0.70); COPD, HR 0.55 (0.34-0.89)] had lower rates of the combined outcome.
Conclusion: In a real-world study, leadless pacemaker patients had lower 2-year complications and reinterventions rates compared with transvenous-VVI pacing in several high-risk subgroups.
Trial registration: ClinicalTrials.gov ID NCT03039712.
Keywords: Complications; High-risk patients; Leadless pacemakers; System reintervention; Transvenous pacemakers.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: S.B. is a consultant for Medtronic, Boston Scientific, Microport, and Zoll. M.F.E.-C. is a consultant for Medtronic, Boston Scientific and Biotronik. L.H., C.L., C.W., K.W., and K.S. are employees and shareholders of Medtronic.
Figures



References
-
- Duray GZ, Ritter P, El-Chami M, Narasimhan C, Omar R, Tolosana JMet al. . Long-term performance of a transcatheter pacing system: 12-month results from the Micra transcatheter pacing study. Heart Rhythm 2017;14:702–9. - PubMed
-
- El-Chami MF, Al-Samadi F, Clementy N, Garweg C, Martinez-Sande JL, Piccini JPet al. . Updated performance of the Micra transcatheter pacemaker in the real-world setting: a comparison to the investigational study and a transvenous historical control. Heart Rhythm 2018;15:1800–7. - PubMed
-
- CMS Manual System . Pub 100-03 Medicare National Coverage Determinations. National Coverage Determination (NCD20.8.4): Leadless Pacemakers. Department of Health & Human Services, Centers for Medicare & Medicaid Services. (23 April 2021, date last accessed).
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

