Dopamine, Immunity, and Disease
- PMID: 36757901
- PMCID: PMC9832385
- DOI: 10.1124/pharmrev.122.000618
Dopamine, Immunity, and Disease
Abstract
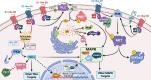
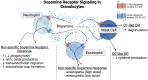
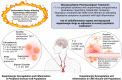
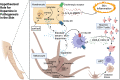
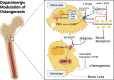
The neurotransmitter dopamine is a key factor in central nervous system (CNS) function, regulating many processes including reward, movement, and cognition. Dopamine also regulates critical functions in peripheral organs, such as blood pressure, renal activity, and intestinal motility. Beyond these functions, a growing body of evidence indicates that dopamine is an important immunoregulatory factor. Most types of immune cells express dopamine receptors and other dopaminergic proteins, and many immune cells take up, produce, store, and/or release dopamine, suggesting that dopaminergic immunomodulation is important for immune function. Targeting these pathways could be a promising avenue for the treatment of inflammation and disease, but despite increasing research in this area, data on the specific effects of dopamine on many immune cells and disease processes remain inconsistent and poorly understood. Therefore, this review integrates the current knowledge of the role of dopamine in immune cell function and inflammatory signaling across systems. We also discuss the current understanding of dopaminergic regulation of immune signaling in the CNS and peripheral tissues, highlighting the role of dopaminergic immunomodulation in diseases such as Parkinson's disease, several neuropsychiatric conditions, neurologic human immunodeficiency virus, inflammatory bowel disease, rheumatoid arthritis, and others. Careful consideration is given to the influence of experimental design on results, and we note a number of areas in need of further research. Overall, this review integrates our knowledge of dopaminergic immunology at the cellular, tissue, and disease level and prompts the development of therapeutics and strategies targeted toward ameliorating disease through dopaminergic regulation of immunity. SIGNIFICANCE STATEMENT: Canonically, dopamine is recognized as a neurotransmitter involved in the regulation of movement, cognition, and reward. However, dopamine also acts as an immune modulator in the central nervous system and periphery. This review comprehensively assesses the current knowledge of dopaminergic immunomodulation and the role of dopamine in disease pathogenesis at the cellular and tissue level. This will provide broad access to this information across fields, identify areas in need of further investigation, and drive the development of dopaminergic therapeutic strategies.
Copyright © 2023 by The Author(s).
Figures





References
-
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ (2010) Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67:139–145. - PubMed
-
- Abreu P, Llorente E, Hernández MM, González MC (1994) Interleukin-1 beta stimulates tyrosine hydroxylase activity in the median eminence. Neuroreport 5:1356–1358. - PubMed
-
- Acharjee S, Branton WG, Vivithanaporn P, Maingat F, Paul AM, Dickie P, Baker GB, Power C (2014) HIV-1 Nef expression in microglia disrupts dopaminergic and immune functions with associated mania-like behaviors. Brain Behav Immun 40:74–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

