Application-specific approaches to MicroCT for evaluation of mouse models of pulmonary disease
- PMID: 36757935
- PMCID: PMC9910664
- DOI: 10.1371/journal.pone.0281452
Application-specific approaches to MicroCT for evaluation of mouse models of pulmonary disease
Abstract
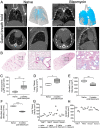
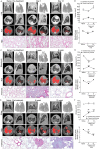
The advent of micro-computed tomography (microCT) has provided significant advancement in our ability to generate clinically relevant assessments of lung health and disease in small animal models. As microCT use to generate outcomes analysis in pulmonary preclinical models has increased there have been substantial improvements in image quality and resolution, and data analysis software. However, there are limited published methods for standardized imaging and automated analysis available for investigators. Manual quantitative analysis of microCT images is complicated by the presence of inflammation and parenchymal disease. To improve the efficiency and limit user-associated bias, we have developed an automated pulmonary air and tissue segmentation (PATS) task list to segment lung air volume and lung tissue volume for quantitative analysis. We demonstrate the effective use of the PATS task list using four distinct methods for imaging, 1) in vivo respiration controlled scanning using a flexiVent, 2) longitudinal breath-gated in vivo scanning in resolving and non-resolving pulmonary disease initiated by lipopolysaccharide-, bleomycin-, and silica-exposure, 3) post-mortem imaging, and 4) ex vivo high-resolution scanning. The accuracy of the PATS task list was compared to manual segmentation. The use of these imaging techniques and automated quantification methodology across multiple models of lung injury and fibrosis demonstrates the broad applicability and adaptability of microCT to various lung diseases and small animal models and presents a significant advance in efficiency and standardization of preclinical microCT imaging and analysis for the field of pulmonary research.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Dekoster K, Decaesteker T, Berghen N, Van den Broucke S, Jonckheere AC, Wouters J, et al. Longitudinal micro-computed tomography-derived biomarkers quantify non-resolving lung fibrosis in a silicosis mouse model. Sci Rep. 2020;10(1):16181. Epub 2020/10/02. doi: 10.1038/s41598-020-73056-6 . - DOI - PMC - PubMed
-
- Rodt T, von Falck C, Dettmer S, Halter R, Maus R, Ask K, et al. Micro-computed tomography of pulmonary fibrosis in mice induced by adenoviral gene transfer of biologically active transforming growth factor-beta1. Respir Res. 2010;11:181. Epub 2010/12/24. doi: 10.1186/1465-9921-11-181 . - DOI - PMC - PubMed
-
- Vande Velde G, De Langhe E, Poelmans J, Dresselaers T, Lories RJ, Himmelreich U. Magnetic resonance imaging for noninvasive assessment of lung fibrosis onset and progression: cross-validation and comparison of different magnetic resonance imaging protocols with micro-computed tomography and histology in the bleomycin-induced mouse model. Invest Radiol. 2014;49(11):691–8. Epub 2014/05/30. doi: 10.1097/RLI.0000000000000071 . - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

