Seroprevalence of anti-Lassa Virus IgG antibodies in three districts of Sierra Leone: A cross-sectional, population-based study
- PMID: 36758101
- PMCID: PMC9946222
- DOI: 10.1371/journal.pntd.0010938
Seroprevalence of anti-Lassa Virus IgG antibodies in three districts of Sierra Leone: A cross-sectional, population-based study
Abstract
Background: Lassa virus (LASV), the cause of the acute viral hemorrhagic illness Lassa fever (LF), is endemic in West Africa. Infections in humans occur mainly after exposure to infected excrement or urine of the rodent-host, Mastomys natalensis. The prevalence of exposure to LASV in Sierra Leone is crudely estimated and largely unknown. This cross-sectional study aimed to establish a baseline point seroprevalence of IgG antibodies to LASV in three administrative districts of Sierra Leone and identify potential risk factors for seropositivity and LASV exposure.
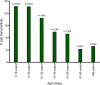
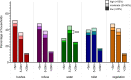
Methodology and principal findings: Between 2015 and 2018, over 10,642 participants from Kenema, Tonkolili, and Port Loko Districts were enrolled in this cross-sectional study. Previous LASV and LF epidemiological studies support classification of these districts as "endemic," "emerging," and "non-endemic", respectively. Dried blood spot samples were tested for LASV antibodies by ELISA to determine the seropositivity of participants, indicating previous exposure to LASV. Surveys were administered to each participant to assess demographic and environmental factors associated with a higher risk of exposure to LASV. Overall seroprevalence for antibodies to LASV was 16.0%. In Kenema, Port Loko, and Tonkolili Districts, seroprevalences were 20.1%, 14.1%, and 10.6%, respectively. In a multivariate analysis, individuals were more likely to be LASV seropositive if they were living in Kenema District, regardless of sex, age, or occupation. Environmental factors contributed to an increased risk of LASV exposure, including poor housing construction and proximity to bushland, forested areas, and refuse.
Conclusions and significance: In this study we determine a baseline LASV seroprevalence in three districts which will inform future epidemiological, ecological, and clinical studies on LF and the LASV in Sierra Leone. The heterogeneity of the distribution of LASV and LF over both space, and time, can make the design of efficacy trials and intervention programs difficult. Having more studies on the prevalence of LASV and identifying potential hyper-endemic areas will greatly increase the awareness of LF and improve targeted control programs related to LASV.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Stenglein MD, Sanders C, Kistler AL, Ruby JG, Franco JY, Reavill DR, et al.. Identification, characterization, and in vitro culture of highly divergent arenaviruses from boa constrictors and annulated tree boas: candidate etiological agents for snake inclusion body disease. MBio. 2012;3(4):e00180–12. doi: 10.1128/mBio.00180-12 - DOI - PMC - PubMed
-
- (WHO) WHO. Lassa fever. Fact sheet.2017; 2020(25-Aug-20). Available from: https://www.who.int/news-room/fact-sheets/detail/lassa-fever.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

