Reduction strategies for inpatient oral third-generation cephalosporins at a cancer center: An interrupted time-series analysis
- PMID: 36758108
- PMCID: PMC9910666
- DOI: 10.1371/journal.pone.0281518
Reduction strategies for inpatient oral third-generation cephalosporins at a cancer center: An interrupted time-series analysis
Abstract
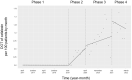
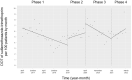
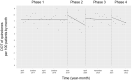
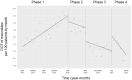
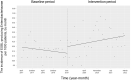
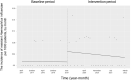
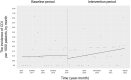
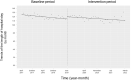
Oral third-generation cephalosporins (3GCs) are not recommended for use owing to their low bioavailability and the risk of emergence of resistant microorganisms with overuse. A standardized and effective method for reducing their use is lacking. Here, in a 60-month, single-institution, interrupted time-series analysis, which was retrospectively conducted between April 1, 2017, and March 31, 2022, we evaluated the effectiveness of a four-phase intervention to reduce the use of 3GCs in patients at a cancer center: Phase 1 (pre-intervention), Phase 2 (review of clinical pathways), Phase 3 (establishment of infectious disease consultation service and implementation of antimicrobial stewardship program), and Phase 4 (educational lecture and pop-up displays for oral antimicrobials at the time of ordering). Although no significant changes were observed in Phases 3 and 4, the first intervention resulted in a significant decrease in the trend and level of days of therapy (DOT) for 3GCs. The level for cephalexin DOT and the trend for sulfamethoxazole-trimethoprim DOT increased in Phase 4, and the trend for amoxicillin and amoxicillin-clavulanate DOT increased in Phase 3. Macrolide DOT showed a decreasing trend in Phases 2 and 4 and decreasing and increased levels in Phases 3 and 4, respectively; no change was observed for quinolones. Actual and adjusted purchase costs of 3GCs decreased significantly during all study periods, while those for oral antimicrobials decreased in Phase 2, and actual purchase costs increased in Phases 3 and 4. No significant reduction in resistant organisms, length of hospital stay, or mortality was observed. This is the first study on the effects of oral 3GC reduction strategies in patients with cancer. We conclude that even facilities that substantially use antimicrobials can efficiently reduce the use of 3GCs.
Copyright: © 2023 Itoh et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Trend of oral antimicrobial use after removal of broad-spectrum antimicrobials from the formulary at a pediatric primary emergency medical center.J Infect Chemother. 2023 May;29(5):502-507. doi: 10.1016/j.jiac.2023.01.002. Epub 2023 Jan 5. J Infect Chemother. 2023. PMID: 36621765
-
Effects of infectious disease consultation and antimicrobial stewardship program at a Japanese cancer center: An interrupted time-series analysis.PLoS One. 2022 Jan 25;17(1):e0263095. doi: 10.1371/journal.pone.0263095. eCollection 2022. PLoS One. 2022. PMID: 35077523 Free PMC article.
-
Impact of targeted intervention using a collaborative approach for oral third-generation cephalosporins: An interrupted time-series analysis.Antimicrob Steward Healthc Epidemiol. 2022 Jul 11;2(1):e115. doi: 10.1017/ash.2022.251. eCollection 2022. Antimicrob Steward Healthc Epidemiol. 2022. PMID: 36483396 Free PMC article.
-
A guide to the treatment of lower respiratory tract infections.Drugs. 1995 Jul;50(1):62-72. doi: 10.2165/00003495-199550010-00006. Drugs. 1995. PMID: 7588090 Review.
-
A critical review of the new oral cephalosporins. Considerations and place in therapy.Arch Fam Med. 1994 Nov;3(11):975-80. doi: 10.1001/archfami.3.11.975. Arch Fam Med. 1994. PMID: 7804480 Review.
Cited by
-
A Population-Based Cohort Study on the Association Between Oral Third-Generation Cephalosporins and Other Antimicrobial Prescriptions and Adverse Events: Findings From the Shizuoka Kokuho Database Study.Cureus. 2025 Feb 12;17(2):e78923. doi: 10.7759/cureus.78923. eCollection 2025 Feb. Cureus. 2025. PMID: 40099084 Free PMC article.
References
-
- World Health Organization Global Action Plan on Antimicrobial Resistance, 2015 [cited 2021 December 25]. Available from: https://www.who.int/antimicrobial-resistance/global-action-plan/en/ - PubMed
-
- The Government of Japan NAP on Antimicrobial Resistance (AMR) 2016–2020 [cited 2021 December 25]. Available from: https://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/000013....
-
- Itoh N, Akazawa N, Kanawaku E, Murakami H, Ishibana Y, Kawamura D, et al.. Effects of infectious disease consultation and antimicrobial stewardship program at a Japanese cancer center: An interrupted time-series analysis. PLOS ONE. 2022;17(1): e0263095. doi: 10.1371/journal.pone.0263095 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

