RhoA rescues cardiac senescence by regulating Parkin-mediated mitophagy
- PMID: 36758801
- PMCID: PMC10020657
- DOI: 10.1016/j.jbc.2023.102993
RhoA rescues cardiac senescence by regulating Parkin-mediated mitophagy
Abstract
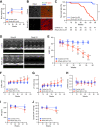
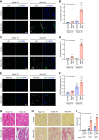
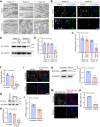
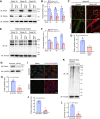
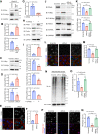
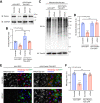
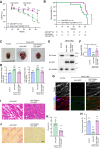
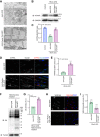
Heart failure is one of the leading causes of death worldwide. RhoA, a small GTPase, governs actin dynamics in various tissue and cell types, including cardiomyocytes; however, its involvement in cardiac function has not been fully elucidated. Here, we generated cardiomyocyte-specific RhoA conditional knockout (cKO) mice, which demonstrated a significantly shorter lifespan with left ventricular dilation and severely impaired ejection fraction. We found that the cardiac tissues of the cKO mice exhibited structural disorganization with fibrosis and also exhibited enhanced senescence compared with control mice. In addition, we show that cardiomyocyte mitochondria were structurally abnormal in the aged cKO hearts. Clearance of damaged mitochondria by mitophagy was remarkably inhibited in both cKO cardiomyocytes and RhoA-knockdown HL-1 cultured cardiomyocytes. In RhoA-depleted cardiomyocytes, we reveal that the expression of Parkin, an E3 ubiquitin ligase that plays a crucial role in mitophagy, was reduced, and expression of N-Myc, a negative regulator of Parkin, was increased. We further reveal that the RhoA-Rho kinase axis induced N-Myc phosphorylation, which led to N-Myc degradation and Parkin upregulation. Re-expression of Parkin in RhoA-depleted cardiomyocytes restored mitophagy, reduced mitochondrial damage, attenuated cardiomyocyte senescence, and rescued cardiac function both in vitro and in vivo. Finally, we found that patients with idiopathic dilated cardiomyopathy without causal mutations for dilated cardiomyopathy showed reduced cardiac expression of RhoA and Parkin. These results suggest that RhoA promotes Parkin-mediated mitophagy as an indispensable mechanism contributing to cardioprotection in the aging heart.
Keywords: Parkin; Ras homolog gene family; cardiomyocyte; cardiomyopathy; member A (RhoA); mitophagy; senescence; signal transduction.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures
References
-
- McMurray J.J., Pfeffer M.A. Heart failure. Lancet. 2005;365:1877–1889. - PubMed
-
- Lam C.S.P., Solomon S.D. Classification of heart failure according to ejection fraction: JACC review topic of the week. J. Am. Coll. Cardiol. 2021;77:3217–3225. - PubMed
-
- Kemp C.D., Conte J.V. The pathophysiology of heart failure. Cardiovasc. Pathol. 2012;21:365–371. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

