Regulating the cell shift of endothelial cell-like myofibroblasts in pulmonary fibrosis
- PMID: 36758986
- PMCID: PMC10249020
- DOI: 10.1183/13993003.01799-2022
Regulating the cell shift of endothelial cell-like myofibroblasts in pulmonary fibrosis
Abstract
Pulmonary fibrosis is a common and severe fibrotic lung disease with high morbidity and mortality. Recent studies have reported a large number of unwanted myofibroblasts appearing in pulmonary fibrosis, and shown that the sustained activation of myofibroblasts is essential for unremitting interstitial fibrogenesis. However, the origin of these myofibroblasts remains poorly understood. Here, we create new mouse models of pulmonary fibrosis and identify a previously unknown population of endothelial cell (EC)-like myofibroblasts in normal lung tissue. We show that these EC-like myofibroblasts significantly contribute myofibroblasts to pulmonary fibrosis, which is confirmed by single-cell RNA sequencing of human pulmonary fibrosis. Using the transcriptional profiles, we identified a small molecule that redirects the differentiation of EC-like myofibroblasts and reduces pulmonary fibrosis in our mouse models. Our study reveals the mechanistic underpinnings of the differentiation of EC-like myofibroblasts in pulmonary fibrosis and may provide new strategies for therapeutic interventions.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: The authors have no potential conflicts of interest to disclose.
Figures
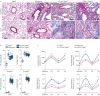
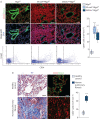
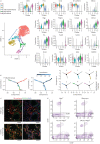

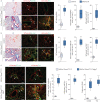



Comment in
-
Endothelial cells in pulmonary fibrosis: more than a bystander.Eur Respir J. 2023 Jun 8;61(6):2300407. doi: 10.1183/13993003.00407-2023. Print 2023 Jun. Eur Respir J. 2023. PMID: 37290810 No abstract available.
-
Reply: Transitioning endothelial cells contribute to pulmonary fibrosis.Eur Respir J. 2023 Sep 28;62(3):2301329. doi: 10.1183/13993003.01329-2023. Print 2023 Sep. Eur Respir J. 2023. PMID: 37770088 Free PMC article.
-
Endothelial to mesenchymal transition: a novel pathological feature of pulmonary fibrosis.Eur Respir J. 2023 Sep 28;62(3):2301178. doi: 10.1183/13993003.01178-2023. Print 2023 Sep. Eur Respir J. 2023. PMID: 37770090 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
