IL-17A-producing CD8+ T cells promote PDAC via induction of inflammatory cancer-associated fibroblasts
- PMID: 36759154
- PMCID: PMC10359545
- DOI: 10.1136/gutjnl-2022-327855
IL-17A-producing CD8+ T cells promote PDAC via induction of inflammatory cancer-associated fibroblasts
Abstract
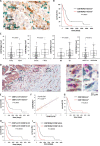
Objective: Pancreatic ductal adenocarcinoma (PDAC) is characterised by an abundant desmoplastic stroma composed of cancer-associated fibroblasts (CAF) and interspersed immune cells. A non-canonical CD8+ T-cell subpopulation producing IL-17A (Tc17) promotes autoimmunity and has been identified in tumours. Here, we evaluated the Tc17 role in PDAC.
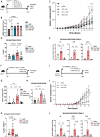
Design: Infiltration of Tc17 cells in PDAC tissue was correlated with patient overall survival and tumour stage. Wild-type (WT) or Il17ra-/- quiescent pancreatic stellate cells (qPSC) were exposed to conditional media obtained from Tc17 cells (Tc17-CM); moreover, co-culture of Tc17-CM-induced inflammatory (i)CAF (Tc17-iCAF) with tumour cells was performed. IL-17A/F-, IL-17RA-, RAG1-deficient and Foxn1nu/nu mice were used to study the Tc17 role in subcutaneous and orthotopic PDAC mouse models.
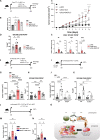
Results: Increased abundance of Tc17 cells highly correlated with reduced survival and advanced tumour stage in PDAC. Tc17-CM induced iCAF differentiation as assessed by the expression of iCAF-associated genes via synergism of IL-17A and TNF. Accordingly, IL-17RA controlled the responsiveness of qPSC to Tc17-CM. Pancreatic tumour cells co-cultured with Tc17-iCAF displayed enhanced proliferation and increased expression of genes implicated in proliferation, metabolism and protection from apoptosis. Tc17-iCAF accelerated growth of mouse and human tumours in Rag1-/- and Foxn1nu/nu mice, respectively. Finally, Il17ra-expressed by fibroblasts was required for Tc17-driven tumour growth in vivo.
Conclusions: We identified Tc17 as a novel protumourigenic CD8+ T-cell subtype in PDAC, which accelerated tumour growth via IL-17RA-dependent stroma modification. We described a crosstalk between three cell types, Tc17, fibroblasts and tumour cells, promoting PDAC progression, which resulted in poor prognosis for patients.
Keywords: cancer immunobiology; cytokines; immune response; inflammatory mechanisms; pancreatic cancer.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



Comment in
-
IL-17A-producing CD8+ T cells in pancreatic cancer.Nat Rev Gastroenterol Hepatol. 2023 Apr;20(4):202. doi: 10.1038/s41575-023-00757-4. Nat Rev Gastroenterol Hepatol. 2023. PMID: 36813843 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
