Pulmonary artery banding in sheep: a novel large animal model for congestive hepatopathy
- PMID: 36759164
- PMCID: PMC10042593
- DOI: 10.1152/japplphysiol.00473.2022
Pulmonary artery banding in sheep: a novel large animal model for congestive hepatopathy
Abstract
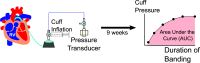
Congestive hepatopathy is becoming increasingly recognized among Fontan-palliated patients. Elevated central venous pressure is thought to drive the pathologic progression, characterized by sinusoidal dilatation, congestion, and fibrosis. A clinically relevant large animal model for congestive hepatopathy would provide a valuable platform for researching novel biomarkers, treatment, and prevention. Here, we report on a titratable, sheep pulmonary artery banding model for this disease application. Pulmonary artery banding was achieved by progressively inflating the implanted pulmonary artery cuff. Right ventricular catheter was implanted to draw venous blood samples and measure pressure. The pulmonary artery cuff pressure served as a surrogate for the intensity of pulmonary artery banding and was measured weekly. After about 9 wk, animals were euthanized, and the liver was harvested for histopathological assessment. Nine animal subjects received pulmonary artery banding for 64 ± 8 days. Four of the nine subjects exhibited moderate to severe liver injury, and three of those four exhibited bridging fibrosis. Increasing pulmonary artery cuff pressure significantly correlated with declining mixed venous oxygen saturation (P = 3.29 × 10-5), and higher congestive hepatic fibrosis score (P = 0.0238), suggesting that pulmonary artery banding strategy can be titrated to achieve right-sided congestion and liver fibrosis. Blood analyses demonstrated an increase in plasma bile acids, aspartate aminotransferase, and γ-glutamyltransferase among subjects with moderate to severe injury, further corroborating liver tissue findings. Our large animal pulmonary artery banding model recapitulates congestive hepatopathy and provides a basis to bridge the current gaps in scientific and clinical understanding about the disease.NEW & NOTEWORTHY We present here a large animal platform for congestive hepatopathy, a disease growing in clinical prevalence due to the increasing number of Fontan-palliated patients. Further data are needed to develop a better clinical management strategy for this poorly characterized patient population. Previous reports of animal models to study this disease have mostly been in small animals with limited fidelity. We show that congestive hepatopathy can be replicated in a chronic, progressive pulmonary artery banding model in sheep. We also show that the banding strategy can be controlled to titrate the level of liver injury. To date, we do not know of any other large animal model that can achieve this level of control over disease phenotype and clinical relevance.
Keywords: Fontan-associated liver disease; congestive hepatopathy; large animal model; liver fibrosis; pulmonary artery banding; sheep.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



References
-
- Wu FM, Kogon B, Earing MG, Aboulhosn JA, Broberg CS, John AS, Harmon A, Sainani NI, Hill AJ, Odze RD, Johncilla ME, Ukomadu C, Gauvreau K, Valente AM, Landzberg MJ; Alliance for Adult Research in Congenital Cardiology (AARCC) Investigators. Liver health in adults with Fontan circulation: a multicenter cross-sectional study. J Thorac Cardiovasc Surg 153: 656–664, 2017. doi:10.1016/j.jtcvs.2016.10.060. - DOI - PubMed
-
- Ukita R, Tipograf Y, Tumen A, Donocoff R, Stokes JW, Foley NM, Talackine J, Cardwell NL, Rosenzweig EB, Cook KE, Bacchetta M. Left pulmonary artery ligation and chronic pulmonary artery banding model for inducing right ventricular-pulmonary hypertension in sheep. ASAIO J 67: e44–e48, 2021. doi:10.1097/MAT.0000000000001197. - DOI - PMC - PubMed
-
- Ukita R, Tumen A, Stokes JW, Pinelli C, Finnie KR, Talackine J, Cardwell NL, Wu WK, Patel Y, Tsai EJ, Rosenzweig EB, Cook KE, Bacchetta M. Progression toward decompensated right ventricular failure in the ovine pulmonary hypertension model. ASAIO J 68: e29–e33, 2022. doi:10.1097/MAT.0000000000001417. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

