Islet Autoantibody Standardization Program: interlaboratory comparison of insulin autoantibody assay performance in 2018 and 2020 workshops
- PMID: 36759347
- PMCID: PMC10036445
- DOI: 10.1007/s00125-023-05877-9
Islet Autoantibody Standardization Program: interlaboratory comparison of insulin autoantibody assay performance in 2018 and 2020 workshops
Abstract
Aims/hypothesis: The Islet Autoantibody Standardization Program (IASP) aims to improve the performance of immunoassays measuring autoantibodies in type 1 diabetes and the concordance of results across laboratories. IASP organises international workshops distributing anonymised serum samples to participating laboratories and centralises the collection and analysis of results. In this report, we describe the results of assays measuring IAA submitted to the IASP 2018 and 2020 workshops.
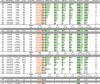
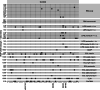
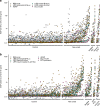
Methods: The IASP distributed uniquely coded sera from individuals with new-onset type 1 diabetes, multiple islet autoantibody-positive individuals, and diabetes-free blood donors in both 2018 and 2020. Serial dilutions of the anti-insulin mouse monoclonal antibody HUI-018 were also included. Sensitivity, specificity, area under the receiver operating characteristic curve (ROC-AUC), partial ROC-AUC at 95% specificity (pAUC95) and concordance of qualitative/quantitative results were compared across assays.
Results: Results from 45 IAA assays of seven different formats and from 37 IAA assays of six different formats were submitted to the IASP in 2018 and 2020, respectively. The median ROC-AUC was 0.736 (IQR 0.617-0.803) and 0.790 (IQR 0.730-0.836), while the median pAUC95 was 0.016 (IQR 0.004-0.021) and 0.023 (IQR 0.014-0.026) in the 2018 and 2020 workshops, respectively. Assays largely differed in AUC (IASP 2018 range 0.232-0.874; IASP 2020 range 0.379-0.924) and pAUC95 (IASP 2018 and IASP 2020 range 0-0.032).
Conclusions/interpretation: Assay formats submitted to this study showed heterogeneous performance. Despite the high variability across laboratories, the in-house radiobinding assay (RBA) remains the gold standard for IAA measurement. However, novel non-radioactive IAA immunoassays showed a good performance and, if further improved, might be considered valid alternatives to RBAs.
Keywords: Autoantibodies; IAA; IASP interlaboratory comparison study; Sensitivity; Specificity; Type 1 diabetes.
© 2023. The Author(s).
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

