Tcf7l2 in hepatocytes regulates de novo lipogenesis in diet-induced non-alcoholic fatty liver disease in mice
- PMID: 36759348
- PMCID: PMC10036287
- DOI: 10.1007/s00125-023-05878-8
Tcf7l2 in hepatocytes regulates de novo lipogenesis in diet-induced non-alcoholic fatty liver disease in mice
Abstract
Aims/hypothesis: Non-alcoholic fatty liver disease (NAFLD) associated with type 2 diabetes may more easily progress towards severe forms of non-alcoholic steatohepatitis (NASH) and cirrhosis. Although the Wnt effector transcription factor 7-like 2 (TCF7L2) is closely associated with type 2 diabetes risk, the role of TCF7L2 in NAFLD development remains unclear. Here, we investigated how changes in TCF7L2 expression in the liver affects hepatic lipid metabolism based on the major risk factors of NAFLD development.
Methods: Tcf7l2 was selectively ablated in the liver of C57BL/6N mice by inducing the albumin (Alb) promoter to recombine Tcf7l2 alleles floxed at exon 5 (liver-specific Tcf7l2-knockout [KO] mice: Alb-Cre;Tcf7l2f/f). Alb-Cre;Tcf7l2f/f and their wild-type (Tcf7l2f/f) littermates were fed a high-fat diet (HFD) or a high-carbohydrate diet (HCD) for 22 weeks to reproduce NAFLD/NASH. Mice were refed a standard chow diet or an HCD to stimulate de novo lipogenesis (DNL) or fed an HFD to provide exogenous fatty acids. We analysed glucose and insulin sensitivity, metabolic respiration, mRNA expression profiles, hepatic triglyceride (TG), hepatic DNL, selected hepatic metabolites, selected plasma metabolites and liver histology.
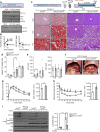
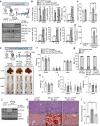
Results: Alb-Cre;Tcf7l2f/f essentially exhibited increased lipogenic genes, but there were no changes in hepatic lipid content in mice fed a normal chow diet. However, following 22 weeks of diet-induced NAFLD/NASH conditions, liver steatosis was exacerbated owing to preferential metabolism of carbohydrate over fat. Indeed, hepatic Tcf7l2 deficiency enhanced liver lipid content in a manner that was dependent on the duration and amount of exposure to carbohydrates, owing to cell-autonomous increases in hepatic DNL. Mechanistically, TCF7L2 regulated the transcriptional activity of Mlxipl (also known as ChREBP) by modulating O-GlcNAcylation and protein content of carbohydrate response element binding protein (ChREBP), and targeted Srebf1 (also called SREBP1) via miRNA (miR)-33-5p in hepatocytes. Eventually, restoring TCF7L2 expression at the physiological level in the liver of Alb-Cre;Tcf7l2f/f mice alleviated liver steatosis without altering body composition under both acute and chronic HCD conditions.
Conclusions/interpretation: In mice, loss of hepatic Tcf7l2 contributes to liver steatosis by inducing preferential metabolism of carbohydrates via DNL activation. Therefore, TCF7L2 could be a promising regulator of the NAFLD associated with high-carbohydrate diets and diabetes since TCF7L2 deficiency may lead to development of NAFLD by promoting utilisation of excess glucose pools through activating DNL.
Data availability: RNA-sequencing data have been deposited into the NCBI GEO under the accession number GSE162449 ( www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE162449 ).
Keywords: Carbohydrate; De novo lipogenesis; Fatty acid; Glucose; Insulin; Lipogenesis; NAFLD; TCF7L2; TG accumulation; Type 2 diabetes.
© 2023. The Author(s).
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

