Behavioral pharmacology of methocinnamox: A potential new treatment for opioid overdose and opioid use disorder
- PMID: 36759567
- PMCID: PMC10281830
- DOI: 10.1002/jeab.831
Behavioral pharmacology of methocinnamox: A potential new treatment for opioid overdose and opioid use disorder
Abstract
Opioid overdose and opioid use disorder continue to be significant public health challenges despite the availability of effective medications and significant efforts at all levels of society. The emergence of highly potent and efficacious opioids such as fentanyl and its derivatives over the last decade has only exacerbated what was already a substantial problem. Behavioral pharmacology research has proven invaluable for understanding the effects of drugs as well as developing and evaluating pharmacotherapies for disorders involving the central nervous system, including substance abuse disorders. This paper describes a program of research characterizing a potent, selective, and long-lasting mu opioid receptor antagonist, methocinnamox, and evaluating its potential for treating opioid overdose and opioid use disorder. Studies in rodents and nonhuman primates demonstrate that methocinnamox prevents and reverses opioid-induced ventilatory depression and selectively blocks opioid self-administration. This work, taken together with rigorous in vitro and ex vivo studies investigating methocinnamox neuropharmacology, lays a solid foundation for the therapeutic utility of this potentially life-saving medication. Moreover, these studies demonstrate how rigorous behavioral pharmacological studies can be integrated in a broader drug discovery and development research program.
Keywords: behavioral pharmacology; methocinnamox; opioid use disorder; opioids; overdose.
© 2023 Society for the Experimental Analysis of Behavior.
Conflict of interest statement
CONFLICT OF INTEREST
David R. Maguire has no known conflict of interest to disclose; Charles P. France is co-holder of a patent for methocinnamox.
Figures



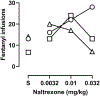

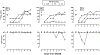
Similar articles
-
Effects of Daily Methocinnamox Treatment on Fentanyl Self-Administration in Rhesus Monkeys.J Pharmacol Exp Ther. 2022 Aug;382(2):181-187. doi: 10.1124/jpet.122.001233. Epub 2022 May 28. J Pharmacol Exp Ther. 2022. PMID: 35643857 Free PMC article.
-
Methocinnamox Reverses and Prevents Fentanyl-Induced Ventilatory Depression in Rats.J Pharmacol Exp Ther. 2021 Apr;377(1):29-38. doi: 10.1124/jpet.120.000387. Epub 2021 Jan 11. J Pharmacol Exp Ther. 2021. PMID: 33431611 Free PMC article.
-
Methocinnamox (MCAM) antagonizes the behavioral suppressant effects of morphine without impairing delayed matching-to-sample accuracy in rhesus monkeys.Psychopharmacology (Berl). 2020 Oct;237(10):3057-3065. doi: 10.1007/s00213-020-05592-y. Epub 2020 Aug 9. Psychopharmacology (Berl). 2020. PMID: 32772146 Free PMC article.
-
Behavioral Pharmacology of Drugs Acting at Mu Opioid Receptors.Handb Exp Pharmacol. 2020;258:127-145. doi: 10.1007/164_2019_265. Handb Exp Pharmacol. 2020. PMID: 31451969 Review.
-
Countermeasures for Preventing and Treating Opioid Overdose.Clin Pharmacol Ther. 2021 Mar;109(3):578-590. doi: 10.1002/cpt.2098. Epub 2020 Nov 29. Clin Pharmacol Ther. 2021. PMID: 33113208 Free PMC article. Review.
Cited by
-
Promoting Reciprocal Relations across Subfields of Behavior Analysis via Collaborations.Perspect Behav Sci. 2023 Jul 26;46(3-4):431-446. doi: 10.1007/s40614-023-00386-x. eCollection 2023 Dec. Perspect Behav Sci. 2023. PMID: 38144552 Free PMC article. Review.
-
Daily methocinnamox treatment dose-dependently attenuates fentanyl self-administration in rhesus monkeys.Neuropharmacology. 2024 Feb 1;243:109777. doi: 10.1016/j.neuropharm.2023.109777. Epub 2023 Nov 8. Neuropharmacology. 2024. PMID: 37944894 Free PMC article.
-
IUPHAR Review: New strategies for medications to treat substance use disorders.Pharmacol Res. 2024 Feb;200:107078. doi: 10.1016/j.phrs.2024.107078. Epub 2024 Jan 20. Pharmacol Res. 2024. PMID: 38246477 Free PMC article. Review.
-
Pharmacology and Toxicology of Opioids-Recent Advances and New Perspectives.Pharmaceuticals (Basel). 2025 Jul 18;18(7):1055. doi: 10.3390/ph18071055. Pharmaceuticals (Basel). 2025. PMID: 40732342 Free PMC article.
References
-
- Adams JU, Paronis CA, & Holtzman SG (1990). Assessment of relative intrinsic activity of mu-opioid analgesics in vivo by using beta-funaltrexamine. The Journal of Pharmacology and Experimental Therapeutics, 255(3), 1027–1032. - PubMed
-
- Ahmad FB, Cisewski JA, Rossen LM, & Sutton P. (2023). Provisional drug overdose death counts. National Center for Health Statistics. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

