Adipocyte-derived extracellular vesicles increase insulin secretion through transport of insulinotropic protein cargo
- PMID: 36759608
- PMCID: PMC9911726
- DOI: 10.1038/s41467-023-36148-1
Adipocyte-derived extracellular vesicles increase insulin secretion through transport of insulinotropic protein cargo
Abstract
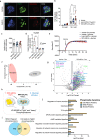
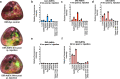
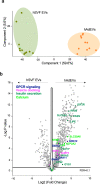
Adipocyte-derived extracellular vesicles (AdEVs) are membranous nanoparticles that convey communication from adipose tissue to other organs. Here, to delineate their role as messengers with glucoregulatory nature, we paired fluorescence AdEV-tracing and SILAC-labeling with (phospho)proteomics, and revealed that AdEVs transfer functional insulinotropic protein cargo into pancreatic β-cells. Upon transfer, AdEV proteins were subjects for phosphorylation, augmented insulinotropic GPCR/cAMP/PKA signaling by increasing total protein abundances and phosphosite dynamics, and ultimately enhanced 1st-phase glucose-stimulated insulin secretion (GSIS) in murine islets. Notably, insulinotropic effects were restricted to AdEVs isolated from obese and insulin resistant, but not lean mice, which was consistent with differential protein loads and AdEV luminal morphologies. Likewise, in vivo pre-treatment with AdEVs from obese but not lean mice amplified insulin secretion and glucose tolerance in mice. This data suggests that secreted AdEVs can inform pancreatic β-cells about insulin resistance in adipose tissue in order to amplify GSIS in times of increased insulin demand.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest related to this study.
Figures




References
-
- van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

