An antibody-drug conjugate targeting GPR56 demonstrates efficacy in preclinical models of colorectal cancer
- PMID: 36759728
- PMCID: PMC10070492
- DOI: 10.1038/s41416-023-02192-3
An antibody-drug conjugate targeting GPR56 demonstrates efficacy in preclinical models of colorectal cancer
Abstract
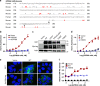
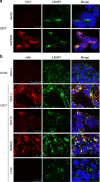
Background: Long-term prognosis remains poor for colorectal cancer (CRC) patients with advanced disease due to treatment resistance. The identification of novel targets is essential for the development of new therapeutic approaches. GPR56, an adhesion GPCR, is highly expressed in CRC tumours and correlates with poor survival. Here, we describe the generation and preclinical evaluation of a novel ADC consisting of an anti-GPR56 antibody (10C7) conjugated with the DNA-damaging payload duocarmycin.
Methods: RNA-seq dataset analysis was performed to determine GPR56 expression in CRC subtypes. The specificity of binding, epitope mapping, and internalisation of 10C7 was examined. 10C7 was conjugated to payload and ADC cytotoxicity was assessed against a panel of CRC cell lines and tumour organoids. Antitumour efficacy was evaluated in xenograft models of CRC cell lines and patient-derived tumours.
Results: High GPR56 was shown to be associated with the microsatellite stable (MSS) subtype that accounts for 80-85% of CRC. GPR56 ADC selectively induced cytotoxicity in CRC cells and tumour organoids at low nanomolar potency in a GPR56-dependent manner and showed significant antitumour efficacy against GPR56-expressing xenograft models.
Conclusions: This study provides the rationale for the future development of a GPR56-targeted ADC approach to potentially treat a large fraction of MSS CRC patients.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Society AC. Cancer Facts & Figures 2021. Atlanta, GA: American Cancer Society; 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

