An optimized bioluminescent substrate for non-invasive imaging in the brain
- PMID: 36759751
- PMCID: PMC10229426
- DOI: 10.1038/s41589-023-01265-x
An optimized bioluminescent substrate for non-invasive imaging in the brain
Abstract
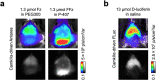
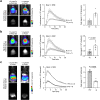
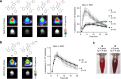
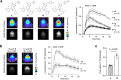
Bioluminescence imaging (BLI) allows non-invasive visualization of cells and biochemical events in vivo and thus has become an indispensable technique in biomedical research. However, BLI in the central nervous system remains challenging because luciferases show relatively poor performance in the brain with existing substrates. Here, we report the discovery of a NanoLuc substrate with improved brain performance, cephalofurimazine (CFz). CFz paired with Antares luciferase produces greater than 20-fold more signal from the brain than the standard combination of D-luciferin with firefly luciferase. At standard doses, Antares-CFz matches AkaLuc-AkaLumine/TokeOni in brightness, while occasional higher dosing of CFz can be performed to obtain threefold more signal. CFz should allow the growing number of NanoLuc-based indicators to be applied to the brain with high sensitivity. Using CFz, we achieve video-rate non-invasive imaging of Antares in brains of freely moving mice and demonstrate non-invasive calcium imaging of sensory-evoked activity in genetically defined neurons.
© 2023. The Author(s).
Conflict of interest statement
J.R.W., M.A.K., M.P.H., L.P.E. and T.A.K. are employees of Promega Corporation and inventors on a patent describing Fz and Fz derivatives. Y.S., L.X.L., X.W., K.M.C., G.H., and M.Z.L. declare no competing interests.
Figures




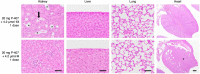
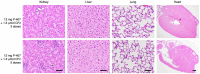

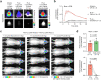


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

