Role of noncoding RNAs in orthodontic tooth movement: new insights into periodontium remodeling
- PMID: 36759852
- PMCID: PMC9912641
- DOI: 10.1186/s12967-023-03951-9
Role of noncoding RNAs in orthodontic tooth movement: new insights into periodontium remodeling
Abstract
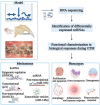
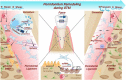
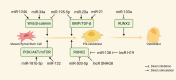
Orthodontic tooth movement (OTM) is biologically based on the spatiotemporal remodeling process in periodontium, the mechanisms of which remain obscure. Noncoding RNAs (ncRNAs), especially microRNAs and long noncoding RNAs, play a pivotal role in maintaining periodontal homeostasis at the transcriptional, post-transcriptional, and epigenetic levels. Under force stimuli, mechanosensitive ncRNAs with altered expression levels transduce mechanical load to modulate intracellular genes. These ncRNAs regulate the biomechanical responses of periodontium in the catabolic, anabolic, and coupling phases throughout OTM. To achieve this, down or upregulated ncRNAs actively participate in cell proliferation, differentiation, autophagy, inflammatory, immune, and neurovascular responses. This review highlights the regulatory mechanism of fine-tuning ncRNAs in periodontium remodeling during OTM, laying the foundation for safe, precise, and personalized orthodontic treatment.
Keywords: Noncoding RNA; Orthodontic tooth movement; Orthodontic treatment; Periodontium remodeling.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Huang H, Williams RC, Kyrkanides S. Accelerated orthodontic tooth movement: molecular mechanisms. Am J Orthod Dentofacial Orthop. 2014;146(5):620–32. - PubMed
-
- Antoun JS, Mei L, Gibbs K, Farella M. Effect of orthodontic treatment on the periodontal tissues. Periodontol 2000. 2017;74(1):140–57. - PubMed
-
- Cattaneo PM, Dalstra M, Melsen B. Strains in periodontal ligament and alveolar bone associated with orthodontic tooth movement analyzed by finite element. Orthod Craniofac Res. 2009;12(2):120–8. - PubMed
-
- Viecilli RF, Katona TR, Chen J, Hartsfield JK, Jr, Roberts WE. Three-dimensional mechanical environment of orthodontic tooth movement and root resorption. Am J Orthod Dentofacial Orthop. 2008;133(6):791e11–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

