A compendium of single extracellular vesicle flow cytometry
- PMID: 36759917
- PMCID: PMC9911638
- DOI: 10.1002/jev2.12299
A compendium of single extracellular vesicle flow cytometry
Abstract
Flow cytometry (FCM) offers a multiparametric technology capable of characterizing single extracellular vesicles (EVs). However, most flow cytometers are designed to detect cells, which are larger than EVs. Whereas cells exceed the background noise, signals originating from EVs partly overlap with the background noise, thereby making EVs more difficult to detect than cells. This technical mismatch together with complexity of EV-containing fluids causes limitations and challenges with conducting, interpreting and reproducing EV FCM experiments. To address and overcome these challenges, researchers from the International Society for Extracellular Vesicles (ISEV), International Society for Advancement of Cytometry (ISAC), and the International Society on Thrombosis and Haemostasis (ISTH) joined forces and initiated the EV FCM working group. To improve the interpretation, reporting, and reproducibility of future EV FCM data, the EV FCM working group published an ISEV position manuscript outlining a framework of minimum information that should be reported about an FCM experiment on single EVs (MIFlowCyt-EV). However, the framework contains limited background information. Therefore, the goal of this compendium is to provide the background information necessary to design and conduct reproducible EV FCM experiments. This compendium contains background information on EVs, the interaction between light and EVs, FCM hardware, experimental design and preanalytical procedures, sample preparation, assay controls, instrument data acquisition and calibration, EV characterization, and data reporting. Although this compendium focuses on EVs, many concepts and explanations could also be applied to FCM detection of other particles within the EV size range, such as bacteria, lipoprotein particles, milk fat globules, and viruses.
Keywords: MIFlowCyt-EV; calibration; extracellular vesicles; flow cytometry; microparticles; nanoparticles; standardization.
© 2023 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
André Görgens has equity interest in and is a consultant for Evox Therapeutics Ltd, Oxford, UK. André Görgens is inventor on patents and patent applications related to extracellular vesicle engineering, manufacturing, and analysis. An Hendrix is an inventor on patents and patent applications related to extracellular vesicle products. Edwin van der Pol is co‐founder and shareholder of Exometry BV. Joshua A. Welsh and Jennifer C. Jones are inventors on NIH patents and patent applications related to extracellular vesicle assays. John P. Nolan is CEO of Cellarcus Biosciences. Joanne Lannigan is a paid consultant for EV development work for Cytek Biosciences. Romaric Lacroix and Françoise Dignat‐George declare that they have patents on extracellular vesicles assays and have received funding from the companies Stago and Beckman Coulter. Xiaomei Yan declares a competing financial interest as a cofounder and shareholder of NanoFCM Inc.
Figures
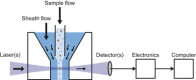


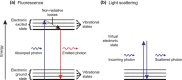
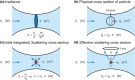
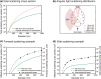
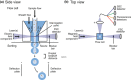




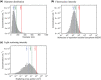




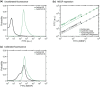


References
-
- Aalberts, M. , van Dissel‐Emiliani, F. M. F. , van Adrichem, N. P. H. , van Wijnen, M. , Wauben, M. H. M. , Stout, T. A. E. , & Stoorvogel, W. (2012). Identification of distinct populations of prostasomes that differentially express prostate stem cell antigen, annexin A1, and GLIPR2 in humans. Biology of Reproduction, 86, 82. - PubMed
-
- Arraud, N. , Gounou, C. , Turpin, D. , & Brisson, A. R. (2016). Fluorescence triggering: A general strategy for enumerating and phenotyping extracellular vesicles by flow cytometry. Cytometry Part A, 89, 184–195. - PubMed
-
- Arraud, N. , Linares, R. , Tan, S. , Gounou, C. , Pasquet, J. M. , Mornet, S. , & Brisson, A. R. (2014). Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. Journal of Thrombosis and Haemostasis, 12, 614–627. - PubMed
-
- Barranco, I. , Padilla, L. , Parrilla, I. , Álvarez‐Barrientos, A. , Pérez‐Patiño, C. , Peña, F. J. , Martínez, E. A. , Rodriguez‐Martínez, H. , & Roca, J. (2019). Extracellular vesicles isolated from porcine seminal plasma exhibit different tetraspanin expression profiles. Scientific Reports, 9, 1. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

