Immunogenicity of decellularized extracellular matrix scaffolds: a bottleneck in tissue engineering and regenerative medicine
- PMID: 36759929
- PMCID: PMC9912640
- DOI: 10.1186/s40824-023-00348-z
Immunogenicity of decellularized extracellular matrix scaffolds: a bottleneck in tissue engineering and regenerative medicine
Abstract
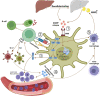
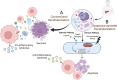
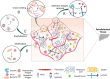
Tissue-engineered decellularized extracellular matrix (ECM) scaffolds hold great potential to address the donor shortage as well as immunologic rejection attributed to cells in conventional tissue/organ transplantation. Decellularization, as the key process in manufacturing ECM scaffolds, removes immunogen cell materials and significantly alleviates the immunogenicity and biocompatibility of derived scaffolds. However, the application of these bioscaffolds still confronts major immunologic challenges. This review discusses the interplay between damage-associated molecular patterns (DAMPs) and antigens as the main inducers of innate and adaptive immunity to aid in manufacturing biocompatible grafts with desirable immunogenicity. It also appraises the impact of various decellularization methodologies (i.e., apoptosis-assisted techniques) on provoking immune responses that participate in rejecting allogenic and xenogeneic decellularized scaffolds. In addition, the key research findings regarding the contribution of ECM alterations, cytotoxicity issues, graft sourcing, and implantation site to the immunogenicity of decellularized tissues/organs are comprehensively considered. Finally, it discusses practical solutions to overcome immunogenicity, including antigen masking by crosslinking, sterilization optimization, and antigen removal techniques such as selective antigen removal and sequential antigen solubilization.
Keywords: Biologic scaffold; Decellularization; Extracellular matrix; Immune response; Recellularization; Tissue engineering; Translational medicine.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Development of decellularized scaffolds for stem cell-driven tissue engineering.J Tissue Eng Regen Med. 2017 Apr;11(4):942-965. doi: 10.1002/term.2061. Epub 2015 Jun 29. J Tissue Eng Regen Med. 2017. PMID: 26119160 Review.
-
Targeted proteomics effectively quantifies differences between native lung and detergent-decellularized lung extracellular matrices.Acta Biomater. 2016 Dec;46:91-100. doi: 10.1016/j.actbio.2016.09.043. Epub 2016 Sep 29. Acta Biomater. 2016. PMID: 27693690 Free PMC article.
-
Decellularization in Tissue Engineering and Regenerative Medicine: Evaluation, Modification, and Application Methods.Front Bioeng Biotechnol. 2022 Apr 25;10:805299. doi: 10.3389/fbioe.2022.805299. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35547166 Free PMC article. Review.
-
Decellularized Extracellular Matrix Scaffolds for Cardiovascular Tissue Engineering: Current Techniques and Challenges.Int J Mol Sci. 2022 Oct 27;23(21):13040. doi: 10.3390/ijms232113040. Int J Mol Sci. 2022. PMID: 36361824 Free PMC article. Review.
-
Preliminary Study on the Antigen-Removal from Extracellular Matrix via Different Decellularization.Tissue Eng Part C Methods. 2022 Jun;28(6):250-263. doi: 10.1089/ten.TEC.2022.0025. Tissue Eng Part C Methods. 2022. PMID: 35596569
Cited by
-
Extracellular matrix-derived materials for tissue engineering and regenerative medicine: A journey from isolation to characterization and application.Bioact Mater. 2024 Jan 17;34:494-519. doi: 10.1016/j.bioactmat.2024.01.004. eCollection 2024 Apr. Bioact Mater. 2024. PMID: 38298755 Free PMC article. Review.
-
Recent Progress of In Vitro 3D Culture of Male Germ Stem Cells.J Funct Biomater. 2023 Nov 8;14(11):543. doi: 10.3390/jfb14110543. J Funct Biomater. 2023. PMID: 37998112 Free PMC article. Review.
-
Antimicrobial Effectiveness Testing of Resorbable Electrospun Fiber Matrix per United States Pharmacopeia (USP) <51>Cureus. 2023 Dec 6;15(12):e50055. doi: 10.7759/cureus.50055. eCollection 2023 Dec. Cureus. 2023. PMID: 38186476 Free PMC article.
-
Standardizing decellularization protocols for optimized uterine tissue bioengineering.Regen Ther. 2024 Dec 21;28:183-190. doi: 10.1016/j.reth.2024.12.011. eCollection 2025 Mar. Regen Ther. 2024. PMID: 39811067 Free PMC article. Review.
-
Pre-vascularized porous gelatin-coated β-tricalcium phosphate scaffolds for bone regeneration: an in vivo and in vitro investigation.In Vitro Cell Dev Biol Anim. 2025 Jan;61(1):67-80. doi: 10.1007/s11626-024-00973-5. Epub 2024 Oct 9. In Vitro Cell Dev Biol Anim. 2025. PMID: 39382735
References
-
- Health Resources and Services Administration. Organ Procurement and Transplantation Network O. National Data Reports, as of March 28, 2022. Available from: http://optn.transplant.hrsa.gov.
-
- Massaro MS, et al. Decellularized xenogeneic scaffolds in transplantation and tissue engineering: Immunogenicity versus positive cell stimulation. Mater Sci Eng C Mater Biol Appl. 2021;127:112203. - PubMed
-
- Sánchez-Fueyo A, Dazzi F. On minor histocompatibility antigens, mixed chimerism, and transplantation tolerance. Am J Transplant. 2021;21(3):919–920. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

