Mycobacterial phosphodiesterase Rv0805 is a virulence determinant and its cyclic nucleotide hydrolytic activity is required for propionate detoxification
- PMID: 36760076
- PMCID: PMC10315211
- DOI: 10.1111/mmi.15030
Mycobacterial phosphodiesterase Rv0805 is a virulence determinant and its cyclic nucleotide hydrolytic activity is required for propionate detoxification
Abstract
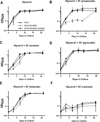
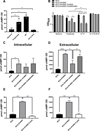
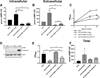
Cyclic AMP (cAMP) signaling is essential to Mycobacterium tuberculosis (Mtb) pathogenesis. However, the roles of phosphodiesterases (PDEs) Rv0805, and the recently identified Rv1339, in cAMP homeostasis and Mtb biology are unclear. We found that Rv0805 modulates Mtb growth within mice, macrophages and on host-associated carbon sources. Mycobacterium bovis BCG grown on a combination of propionate and glycerol as carbon sources showed high levels of cAMP and had a strict requirement for Rv0805 cNMP hydrolytic activity. Supplementation with vitamin B12 or spontaneous genetic mutations in the pta-ackA operon restored the growth of BCGΔRv0805 and eliminated propionate-associated cAMP increases. Surprisingly, reduction of total cAMP levels by ectopic expression of Rv1339 restored only 20% of growth, while Rv0805 complementation fully restored growth despite a smaller effect on total cAMP levels. Deletion of an Rv0805 localization domain also reduced BCG growth in the presence of propionate and glycerol. We propose that localized Rv0805 cAMP hydrolysis modulates activity of a specialized pathway associated with propionate metabolism, while Rv1339 has a broader role in cAMP homeostasis. Future studies will address the biological roles of Rv0805 and Rv1339, including their impacts on metabolism, cAMP signaling and Mtb pathogenesis.
Keywords: TB complex bacteria; cAMP signaling; carbon metabolism; cyclic nucleotide phosphodiesterase.
© 2023 John Wiley & Sons Ltd.
Figures


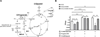

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

