Efficacy comparison of 3CL protease inhibitors ensitrelvir and nirmatrelvir against SARS-CoV-2 in vitro and in vivo
- PMID: 36760083
- PMCID: PMC10068418
- DOI: 10.1093/jac/dkad027
Efficacy comparison of 3CL protease inhibitors ensitrelvir and nirmatrelvir against SARS-CoV-2 in vitro and in vivo
Abstract
Objectives: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become established in the human population, making the need to develop safe and effective treatments critical. We have developed the small-molecule antiviral ensitrelvir, which targets the 3C-like (3CL) protease of SARS-CoV-2. This study evaluated the in vitro and in vivo efficacy of ensitrelvir compared with that of another SARS-CoV-2 3CL PI, nirmatrelvir.
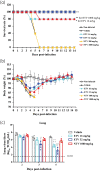
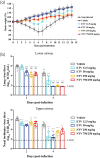
Methods: Cultured cells, BALB/cAJcl mice and Syrian hamsters were infected with various SARS-CoV-2 strains, including the ancestral strain WK-521, mouse-adapted SARS-CoV-2 (MA-P10) strain, Delta strain and Omicron strain. Ensitrelvir efficacy was compared with that of nirmatrelvir. Effective concentrations were determined in vitro based on virus-induced cytopathic effects, viral titres and RNA levels. Lung viral titres, nasal turbinate titres, body-weight changes, and animal survival were also monitored.
Results: Ensitrelvir and nirmatrelvir showed comparable antiviral activity in multiple cell lines. Both ensitrelvir and nirmatrelvir reduced virus levels in the lungs of mice and the nasal turbinates and lungs of hamsters. However, ensitrelvir demonstrated comparable or better in vivo efficacy than that of nirmatrelvir when present at similar or slightly lower unbound-drug plasma concentrations.
Conclusions: Direct in vitro and in vivo efficacy comparisons of 3CL PIs revealed that ensitrelvir demonstrated comparable in vitro efficacy to that of nirmatrelvir in cell culture and exhibited equal to or greater in vivo efficacy in terms of unbound-drug plasma concentration in both animal models evaluated. The results suggest that ensitrelvir may become an important resource for treating individuals infected with SARS-CoV-2.
© The Author(s) 2023. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

