ΔNp63α-mediated epigenetic regulation in keratinocyte senescence
- PMID: 36760085
- PMCID: PMC9980457
- DOI: 10.1080/15592294.2023.2173931
ΔNp63α-mediated epigenetic regulation in keratinocyte senescence
Abstract
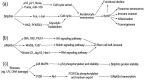
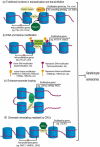
Keratinocyte senescence contributes to skin ageing and epidermal dysfunction. According to the existing knowledge, the transcription factor ΔNp63α plays pivotal roles in differentiation and proliferation of keratinocytes. It is traditionally accepted that ΔNp63α exerts its functions via binding to promoter regions to activate or repress gene transcription. However, accumulating evidence demonstrates that ΔNp63α can bind to elements away from promoter regions of its target genes, mediating epigenetic regulation. On the other hand, several epigenetic alterations, including DNA methylation, histone modification and variation, chromatin remodelling, as well as enhancer-promoter looping, are found to be related to cell senescence. To systematically elucidate how ΔNp63α affects keratinocyte senescence via epigenetic regulation, we comprehensively compiled the literatures on the roles of ΔNp63α in keratinocyte senescence, epigenetics in cellular senescence, and the relation between ΔNp63α-mediated epigenetic regulation and keratinocyte senescence. Based on the published data, we conclude that ΔNp63α mediates epigenetic regulation via multiple mechanisms: recruiting epigenetic enzymes to modify DNA or histones, coordinating chromatin remodelling complexes (CRCs) or regulating their expression, and mediating enhancer-promoter looping. Consequently, the expression of genes related to cell cycle is modulated, and proliferation of keratinocytes and renewal of stem cells are maintained, by ΔNp63α. During skin inflammaging, the decline of ΔNp63α may lead to epigenetic dysregulation, resultantly deteriorating keratinocyte senescence.
Keywords: Keratinocyte; cell proliferation; cellular senescence; chromatin remodeling; epigenetic regulation; skin aging; ΔNp63α.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
