Modeling lymphangiogenesis: Pairing in vitro and in vivo metrics
- PMID: 36760223
- PMCID: PMC10121924
- DOI: 10.1111/micc.12802
Modeling lymphangiogenesis: Pairing in vitro and in vivo metrics
Abstract
Lymphangiogenesis is the mechanism by which the lymphatic system develops and expands new vessels facilitating fluid drainage and immune cell trafficking. Models to study lymphangiogenesis are necessary for a better understanding of the underlying mechanisms and to identify or test new therapeutic agents that target lymphangiogenesis. Across the lymphatic literature, multiple models have been developed to study lymphangiogenesis in vitro and in vivo. In vitro, lymphangiogenesis can be modeled with varying complexity, from monolayers to hydrogels to explants, with common metrics for characterizing proliferation, migration, and sprouting of lymphatic endothelial cells (LECs) and vessels. In comparison, in vivo models of lymphangiogenesis often use genetically modified zebrafish and mice, with in situ mouse models in the ear, cornea, hind leg, and tail. In vivo metrics, such as activation of LECs, number of new lymphatic vessels, and sprouting, mirror those most used in vitro, with the addition of lymphatic vessel hyperplasia and drainage. The impacts of lymphangiogenesis vary by context of tissue and pathology. Therapeutic targeting of lymphangiogenesis can have paradoxical effects depending on the pathology including lymphedema, cancer, organ transplant, and inflammation. In this review, we describe and compare lymphangiogenic outcomes and metrics between in vitro and in vivo studies, specifically reviewing only those publications in which both testing formats are used. We find that in vitro studies correlate well with in vivo in wound healing and development, but not in the reproductive tract or the complex tumor microenvironment. Considerations for improving in vitro models are to increase complexity with perfusable microfluidic devices, co-cultures with tissue-specific support cells, the inclusion of fluid flow, and pairing in vitro models of differing complexities. We believe that these changes would strengthen the correlation between in vitro and in vivo outcomes, giving more insight into lymphangiogenesis in healthy and pathological states.
Keywords: in vitro models; lymphatics; regenerative medicine.
© 2023 The Authors. Microcirculation published by John Wiley & Sons Ltd.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interests.
Figures


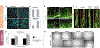
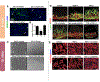

References
-
- Joukov V; Pajusola K; Kaipainen A; Chilov D; Lahtinen I; Kukk E; Saksela O; Kalkkinen N; Alitalo K A Novel Vascular Endothelial Growth Factor, VEGF-C, Is a Ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) Receptor Tyrosine Kinases. EMBO J. 1996, 15 (2), 290–298. 10.1002/j.1460-2075.1996.tb00359.x. - DOI - PMC - PubMed
-
- Rissanen TT; Markkanen JE; Gruchala M; Heikura T; Puranen A; Kettunen MI; Kholová I; Kauppinen RA; Achen MG; Stacker SA; Alitalo K; Ylä-Herttuala S VEGF-D Is the Strongest Angiogenic and Lymphangiogenic Effector Among VEGFs Delivered Into Skeletal Muscle via Adenoviruses. Circ. Res. 2003, 92 (10), 1098–1106. 10.1161/01.RES.0000073584.46059.E3. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

