Communication of gut microbiota and brain via immune and neuroendocrine signaling
- PMID: 36760508
- PMCID: PMC9907780
- DOI: 10.3389/fmicb.2023.1118529
Communication of gut microbiota and brain via immune and neuroendocrine signaling
Abstract
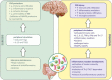
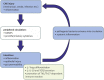
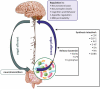
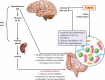
The gastrointestinal tract of the human is inhabited by about 5 × 1013 bacteria (of about 1,000 species) as well as archaea, fungi, and viruses. Gut microbiota is known to influence the host organism, but the host may also affect the functioning of the microbiota. This bidirectional cooperation occurs in three main inter-organ signaling: immune, neural, and endocrine. Immune communication relies mostly on the cytokines released by the immune cells into circulation. Also, pathogen-associated or damage-associated molecular patterns (PAMPs or DAMPs) may enter circulation and affect the functioning of the internal organs and gut microbiota. Neural communication relies mostly on the direct anatomical connections made by the vagus nerve, or indirect connections via the enteric nervous system. The third pathway, endocrine communication, is the broadest one and includes the hypothalamic-pituitary-adrenal axis. This review focuses on presenting the latest data on the role of the gut microbiota in inter-organ communication with particular emphasis on the role of neurotransmitters (catecholamines, serotonin, gamma-aminobutyric acid), intestinal peptides (cholecystokinin, peptide YY, and glucagon-like peptide 1), and bacterial metabolites (short-chain fatty acids).
Keywords: HPA axis; gut microbiota; gut-brain axis; immune system; vagus nerve.
Copyright © 2023 Kasarello, Cudnoch-Jedrzejewska and Czarzasta.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Akbari E., Asemi Z., Daneshvar Kakhaki R., Bahmani F., Kouchaki E., Tamtaji O. R., et al. (2016). Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: A randomized, double-blind and controlled trial. Front. Aging Neurosci. 8:256. 10.3389/fnagi.2016.00256 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

