Inhibition of human oral squamous cell carcinoma proliferation and migration by prodrug-activating suicide gene therapies
- PMID: 36761002
- PMCID: PMC9905654
- DOI: 10.3892/etm.2023.11790
Inhibition of human oral squamous cell carcinoma proliferation and migration by prodrug-activating suicide gene therapies
Erratum in
-
Erratum: [Corrigendum] Inhibition of human oral squamous cell carcinoma proliferation and migration by prodrug‑activating suicide gene therapies.Exp Ther Med. 2023 Aug 7;26(4):460. doi: 10.3892/etm.2023.12159. eCollection 2023 Oct. Exp Ther Med. 2023. PMID: 37664682 Free PMC article.
Abstract
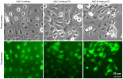
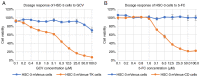
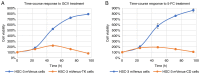
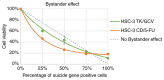
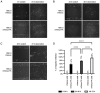
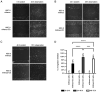
Head and neck squamous cell carcinoma (HNSCC), which originates from mucosal epithelium in the oral cavity, pharynx and larynx, is the sixth most common malignancy in the world. The prognosis of HNSCC is not satisfactory due to metastasis, resulting in 5-year survival rates ranging from 65.9 to 67.2%. Previously, we developed a method to evaluate the effect prodrug-activating suicide gene (PA-SG) therapy on the proliferation of HNSCC. The present study investigated PA-SG therapy on metastatic HNSCC by wound-healing assay and our previously established method. HSC-3 cells with stable expression of suicide genes thymidine kinase (TK) or cytosine deaminase (CD) were treated with prodrugs ganciclovir (GCV) or 5-fluorocytosine (5-FC), respectively. Both GCV and 5-FC inhibited HSC-3 proliferation while the bystander effect of CD/5-FC was greater compared with that of TK/GCV. GCV showed a greater anti-migration effect compared with that of 5-FC. To the best of our knowledge, the present study is the first to evaluate the anti-migratory and anti-proliferative effects of PA-SG therapies on metastatic HNSCC. This may also serve as a general method to quantify other types of PA-SC therapy. The present results demonstrated that PA-SG therapy is a promising treatment for anti-metastatic HNSCC therapy development.
Keywords: cytosine deaminase and 5-fluorocytosine; head and neck squamous cell carcinoma; metastasis; prodrug; suicide gene therapy; thymidine kinase and ganciclovir.
Copyright: © Xu et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Combined antitumor effects of an adenoviral cytosine deaminase/thymidine kinase fusion gene in rat C6 glioma.Neurosurgery. 2000 Oct;47(4):931-8; discussion 938-9. doi: 10.1097/00006123-200010000-00026. Neurosurgery. 2000. PMID: 11014433
-
Comparison of gene therapy with the herpes simplex virus thymidine kinase gene and the bacterial cytosine deaminase gene for the treatment of hepatocellular carcinoma.Scand J Gastroenterol. 1999 Oct;34(10):1033-41. doi: 10.1080/003655299750025156. Scand J Gastroenterol. 1999. PMID: 10563675
-
Quantitative evaluation and comparison of two prodrug-activating suicide gene therapies on oral squamous cell carcinoma.Am J Cancer Res. 2021 Apr 15;11(4):1672-1682. eCollection 2021. Am J Cancer Res. 2021. PMID: 33948381 Free PMC article.
-
Therapeutic potential of stem cells expressing suicide genes that selectively target human breast cancer cells: evidence that they exert tumoricidal effects via tumor tropism (review).Int J Oncol. 2012 Sep;41(3):798-804. doi: 10.3892/ijo.2012.1523. Epub 2012 Jun 20. Int J Oncol. 2012. PMID: 22736197 Free PMC article. Review.
-
Advances in imaging gene-directed enzyme prodrug therapy.Curr Pharm Biotechnol. 2011 Apr;12(4):497-507. doi: 10.2174/138920111795163896. Curr Pharm Biotechnol. 2011. PMID: 21342105 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
