Safety of intraocular anti-VEGF antibody treatment under in vitro HTLV-1 infection
- PMID: 36761168
- PMCID: PMC9905742
- DOI: 10.3389/fimmu.2022.1089286
Safety of intraocular anti-VEGF antibody treatment under in vitro HTLV-1 infection
Abstract
Introduction: HTLV-1 (human T-cell lymphotropic virus type 1) is a retrovirus that infects approximately 20 million people worldwide. Many diseases are caused by this virus, including HTLV-1-associated myelopathy, adult T-cell leukemia, and HTLV-1 uveitis. Intraocular anti-vascular endothelial growth factor (VEGF) antibody injection has been widely used in ophthalmology, and it is reportedly effective against age-related macular degeneration, complications of diabetic retinopathy, and retinal vein occlusions. HTLV-1 mimics VEGF165, the predominant isoform of VEGF, to recruit neuropilin-1 and heparan sulfate proteoglycans. VEGF165 is also a selective competitor of HTLV-1 entry. Here, we investigated the effects of an anti-VEGF antibody on ocular status under conditions of HTLV-1 infection in vitro.
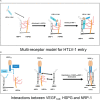
Methods: We used MT2 and TL-Om1 cells as HTLV-1-infected cells and Jurkat cells as controls. Primary human retinal pigment epithelial cells (HRPEpiCs) and ARPE19 HRPEpiCs were used as ocular cells; MT2/TL-Om1/Jurkat cells and HRPEpiCs/ARPE19 cells were co-cultured to simulate the intraocular environment of HTLV-1-infected patients. Aflibercept was administered as an anti-VEGF antibody. To avoid possible T-cell adhesion, we lethally irradiated MT2/TL-Om1/Jurkat cells prior to the experiments.
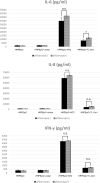
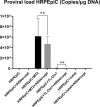
Results: Anti-VEGF antibody treatment had no effect on activated NF-κB production, inflammatory cytokines, chemokines, HTLV-1 proviral load (PVL), or cell counts in the retinal pigment epithelium (RPE) under MT2 co-culture conditions. Under TL-Om1 co-culture conditions, anti-VEGF antibody treatment did not affect the production of activated NF-κB, chemokines, PVL, or cell counts, but production of the inflammatory cytokine IL-6 was increased. In addition, anti-VEGF treatment did not affect PVL in HTLV-1-infected T cells.
Conclusion: This preliminary in vitro assessment indicates that intraocular anti-VEGF antibody treatment for HTLV-1 infection does not exacerbate HTLV-1-related inflammation and thus may be safe for use.
Keywords: HTLV-1 uveitis; VEGF; aflibercept; human T-cell leukemia virus type 1; ocular inflammation; uveitis.
Copyright © 2023 Zong, Kamoi, Kurozumi-Karube, Zhang, Yang and Ohno-Matsui.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer MM declared a shared affiliation with the authors to the handling editor at the time of review.
Figures




Similar articles
-
In vitro Evaluation of the Safety of Adalimumab for the Eye Under HTLV-1 Infection Status: A Preliminary Study.Front Microbiol. 2020 Dec 23;11:522579. doi: 10.3389/fmicb.2020.522579. eCollection 2020. Front Microbiol. 2020. PMID: 33424777 Free PMC article.
-
Safety of Infliximab for the Eye Under Human T-Cell Leukemia Virus Type 1 Infectious Conditions in vitro.Front Microbiol. 2019 Sep 18;10:2148. doi: 10.3389/fmicb.2019.02148. eCollection 2019. Front Microbiol. 2019. PMID: 31620105 Free PMC article.
-
Tropical spastic paraparesis and HTLV-1 associated myelopathy: clinical, epidemiological, virological and therapeutic aspects.Rev Neurol (Paris). 2012 Mar;168(3):257-69. doi: 10.1016/j.neurol.2011.12.006. Epub 2012 Mar 7. Rev Neurol (Paris). 2012. PMID: 22405461 Review.
-
[Intraocular inflammation and homeostasis of the eye].Nippon Ganka Gakkai Zasshi. 2009 Mar;113(3):344-77; discussion 378. Nippon Ganka Gakkai Zasshi. 2009. PMID: 19348183 Review. Japanese.
-
High IFN-γ/IL-10 expression ratio and increased frequency of persistent human T-cell lymphotropic virus type 1-infected clones are associated with human T-cell lymphotropic virus type 1-associated myelopathy/tropical spastic paraparesis development.Intervirology. 2015;58(2):106-14. doi: 10.1159/000371766. Epub 2015 Mar 27. Intervirology. 2015. PMID: 25833232
Cited by
-
Applications of Biological Therapy for Latent Infections: Benefits and Risks.Int J Mol Sci. 2024 Aug 24;25(17):9184. doi: 10.3390/ijms25179184. Int J Mol Sci. 2024. PMID: 39273134 Free PMC article. Review.
-
Impact of genetic alterations on central nervous system progression of primary vitreoretinal lymphoma.Haematologica. 2024 Nov 1;109(11):3641-3649. doi: 10.3324/haematol.2023.284953. Haematologica. 2024. PMID: 38841798 Free PMC article.
-
Ophthalmic Use of Targeted Biologics in the Management of Intraocular Diseases: Current and Emerging Therapies.Antibodies (Basel). 2024 Oct 11;13(4):86. doi: 10.3390/antib13040086. Antibodies (Basel). 2024. PMID: 39449328 Free PMC article. Review.
-
The tyrosine kinase KDR is essential for the survival of HTLV-1-infected T cells by stabilizing the Tax oncoprotein.Nat Commun. 2024 Jun 25;15(1):5380. doi: 10.1038/s41467-024-49737-5. Nat Commun. 2024. PMID: 38918393 Free PMC article.
-
Ocular Toxoplasmosis: Advances in Toxoplasma gondii Biology, Clinical Manifestations, Diagnostics, and Therapy.Pathogens. 2024 Oct 14;13(10):898. doi: 10.3390/pathogens13100898. Pathogens. 2024. PMID: 39452769 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

