Effects of the therapy shift from cortisone acetate to modified-release hydrocortisone in a group of patients with adrenal insufficiency
- PMID: 36761196
- PMCID: PMC9902698
- DOI: 10.3389/fendo.2023.1093838
Effects of the therapy shift from cortisone acetate to modified-release hydrocortisone in a group of patients with adrenal insufficiency
Abstract
Objective: Patients with adrenal insufficiency (AI) may be exposed to supraphysiological glucocorticoids levels during standard treatment with cortisone acetate (CA) or immediate-release hydrocortisone (IR-HC). Recent studies, predominantly including patients in IR-HC treatment, suggested that modified-release hydrocortisone (MRH) provide a more physiological cortisol rhythm, improving metabolic control and quality of life. Our primary aim was to assess clinical and biochemical modifications in patients shifted from CA to MRH.
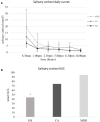
Design/methods: We designed a retrospective longitudinal study, enrolling 45 AI patients (22 primary and 23 secondary AI) treated exclusively with CA thrice daily, shifted to MRH once daily; 29/45 patients concluded at least 18-months follow-up (MRH-group). We recruited 35 AI patients continuing CA as a control group (CA-group). Biochemical and clinical data, including metabolic parameters, bone quality, and symptoms of under- or overtreatment were collected. In 24 patients, a daily salivary cortisol curve (SCC) performed before and one month after shifting to MRH was compared to healthy subjects (HS).
Results: No significant changes in glycometabolic and bone parameters were observed both in MRH and CA-groups during a median follow-up of 35 months. A more frequent decrease in blood pressure values (23.1% vs 2.8%, p=0.04) and improvement of under- or overtreatment symptoms were observed in MRH vs CA-group. The SCC showed a significant steroid overexposure in both CA and MRH-groups compared to HS [AUC (area under the curve) = 74.4 ± 38.1 nmol×hr/L and 94.6 ± 62.5 nmol×hr/L respectively, vs 44.1 ± 8.4 nmol×hr/L, p<0.01 for both comparisons], although SCC profile was more similar to HS in MRH-group.
Conclusions: In our experience, patients shifted from CA to equivalent doses of MRH do not show significant glycometabolic modifications but blood pressure control and symptoms of over-or undertreatment may improve. The lack of amelioration in glucose metabolism and total cortisol daily exposure could suggest the need for a dose reduction when shifting from CA to MRH, due to their different pharmacokinetics.
Keywords: Addison disease; adrenal insufficiency; hydrocortisone; modified-release hydrocortisone (MRH); salivary cortisol.
Copyright © 2023 Frigerio, Carosi, Ferrante, Sala, Polledri, Fustinoni, Ambrosi, Chiodini, Mantovani, Morelli and Arosio.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Preliminary results from whole-genome expression analysis in patients with secondary adrenal insufficiency treated with modified-release hydrocortisone.Endocrine. 2021 Jul;73(1):177-185. doi: 10.1007/s12020-020-02578-w. Epub 2021 Jan 8. Endocrine. 2021. PMID: 33417142
-
Dual-release hydrocortisone improves body composition and the glucometabolic profile in patients with secondary adrenal insufficiency.Endocrine. 2024 Jun;84(3):1182-1192. doi: 10.1007/s12020-024-03711-9. Epub 2024 Feb 12. Endocrine. 2024. PMID: 38345683 Free PMC article.
-
Monitoring adrenal insufficiency through salivary steroids: a pilot study.Eur J Endocrinol. 2024 Mar 30;190(4):327-337. doi: 10.1093/ejendo/lvae037. Eur J Endocrinol. 2024. PMID: 38571387
-
Glucocorticoid replacement regimens for treating congenital adrenal hyperplasia.Cochrane Database Syst Rev. 2020 Mar 19;3(3):CD012517. doi: 10.1002/14651858.CD012517.pub2. Cochrane Database Syst Rev. 2020. PMID: 32190901 Free PMC article.
-
Secondary adrenal insufficiency: from the physiopathology to the possible role of modified-release hydrocortisone treatment.Minerva Endocrinol. 2018 Jun;43(2):183-197. doi: 10.23736/S0391-1977.17.02701-8. Epub 2017 Jul 27. Minerva Endocrinol. 2018. PMID: 28750490 Review.
Cited by
-
Extended-release Hydrocortisone Formulations-Is There a Clinically Meaningful Benefit?J Clin Endocrinol Metab. 2025 Feb 18;110(3):e566-e573. doi: 10.1210/clinem/dgae822. J Clin Endocrinol Metab. 2025. PMID: 39656185 Free PMC article. Review.
References
-
- Giordano R, Guaraldi F, Marinazzo E, Fumarola F, Rampino A, Berardelli R, et al. . Improvement of anthropometric and metabolic parameters, and quality of life following treatment with dual-release hydrocortisone in patients with addison’s disease. Endocrine (2016) 51(2):360–8. doi: 10.1007/s12020-015-0681-z - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

