Molecular-Level Insight into Charge Carrier Transport and Speciation in Solid Polymer Electrolytes by Chemically Tuning Both Polymer and Lithium Salt
- PMID: 36761231
- PMCID: PMC9900585
- DOI: 10.1021/acs.jpcc.2c07032
Molecular-Level Insight into Charge Carrier Transport and Speciation in Solid Polymer Electrolytes by Chemically Tuning Both Polymer and Lithium Salt
Abstract
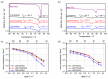
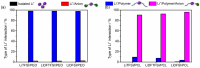
The advent of Li-metal batteries has seen progress toward studies focused on the chemical modification of solid polymer electrolytes, involving tuning either polymer or Li salt properties to enhance the overall cell performance. This study encompasses chemically modifying simultaneously both polymer matrix and lithium salt by assessing ion coordination environments, ion transport mechanisms, and molecular speciation. First, commercially used lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) salt is taken as a reference, where F atoms become partially substituted by one or two H atoms in the -CF3 moieties of LiTFSI. These substitutions lead to the formation of lithium(difluoromethanesulfonyl)(trifluoromethanesulfonyl)imide (LiDFTFSI) and lithium bis(difluoromethanesulfonyl)imide (LiDFSI) salts. Both lithium salts promote anion immobilization and increase the lithium transference number. Second, we show that exchanging archetypal poly(ethylene oxide) (PEO) with poly(ε-caprolactone) (PCL) significantly changes charge carrier speciation. Studying the ionic structures of these polymer/Li salt combinations (LiTFSI, LiDFTFSI or LiDFSI with PEO or PCL) by combining molecular dynamics simulations and a range of experimental techniques, we provide atomistic insights to understand the solvation structure and synergistic effects that impact macroscopic properties, such as Li+ conductivity and transference number.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Nanoscale Ion Transport Enhances Conductivity in Solid Polymer-Ceramic Lithium Electrolytes.ACS Nano. 2024 Jan 30;18(4):2750-2762. doi: 10.1021/acsnano.3c03901. Epub 2024 Jan 4. ACS Nano. 2024. PMID: 38174956
-
Synergistic theoretical and experimental study on the ion dynamics of bis(trifluoromethanesulfonyl)imide-based alkali metal salts for solid polymer electrolytes.Phys Chem Chem Phys. 2023 Sep 20;25(36):25038-25054. doi: 10.1039/d3cp02989a. Phys Chem Chem Phys. 2023. PMID: 37698851
-
Self-Standing Single-Ion Borate Salt-Based Polymer Electrolyte for Lithium Metal Batteries.ACS Appl Mater Interfaces. 2024 Sep 18;16(37):48792-48802. doi: 10.1021/acsami.3c15703. Epub 2023 Dec 27. ACS Appl Mater Interfaces. 2024. PMID: 38149481 Review.
-
Investigating the Effects of Lithium Phosphorous Oxynitride Coating on Blended Solid Polymer Electrolytes.ACS Appl Mater Interfaces. 2020 Sep 9;12(36):40749-40758. doi: 10.1021/acsami.0c09113. Epub 2020 Aug 27. ACS Appl Mater Interfaces. 2020. PMID: 32786244 Free PMC article.
-
Comprehensive Review of Polymer Architecture for All-Solid-State Lithium Rechargeable Batteries.Materials (Basel). 2020 May 29;13(11):2488. doi: 10.3390/ma13112488. Materials (Basel). 2020. PMID: 32486029 Free PMC article. Review.
References
-
- Yang G.; Chanthad C.; Oh H.; Ayhan I. A.; Wang Q. Organic-Inorganic Hybrid Electrolytes from Ionic Liquid-Functionalized Octasilsesquioxane for Lithium Metal Batteries. J. Mater. Chem. A Mater. 2017, 5 (34), 18012–18019. 10.1039/C7TA04599A. - DOI
-
- Liu B.; Zhang J. G.; Xu W. Advancing Lithium Metal Batteries. Joule. 2018, 2, 833–845. 10.1016/j.joule.2018.03.008. - DOI
-
- Korthauer R.Lithium-Ion Batteries: Basics and Applications; Springer: Berlin, 2018.
-
- Varzi A.; Raccichini R.; Passerini S.; Scrosati B. Challenges and Prospects of the Role of Solid Electrolytes in the Revitalization of Lithium Metal Batteries. J. Mater. Chem. A Mater. 2016, 4 (44), 17251–17259. 10.1039/C6TA07384K. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
