Real-world effect of bevacizumab and eribulin on metastatic breast cancer using a propensity score matching analysis
- PMID: 36761387
- PMCID: PMC9892966
- DOI: 10.3892/mco.2023.2608
Real-world effect of bevacizumab and eribulin on metastatic breast cancer using a propensity score matching analysis
Abstract
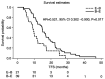
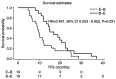
Bevacizumab and eribulin are novel agents for the treatment of HER2-negative metastatic breast cancer (MBC); however, the choice between bevacizumab and eribulin for MBC can be difficult. The present study aimed to compare two treatment strategies, eribulin followed by bevacizumab and paclitaxel (BEV + PTX) versus BEV + PTX followed by eribulin, to determine whether the order of administration affects the outcome of MBC in the real world. A total of 180 patients who started BEV + PTX and eribulin treatment for HER2-negative MBC from August 2011 to June 2018 were selected. Of these, 84 patients were treated with both BEV + PTX and eribulin sequentially. To evaluate the influence of the sequential order, the efficacy of BEV + PTX followed by eribulin (B-E arm) was compared to treatment with the reverse sequence (E-B arm). The propensity score matching method (PSMA) was used to improve the robustness of the findings from the present study. A total of 60 cases analyzed received BEV + PTX or eribulin as either first- or second-line treatment. In the entire cohort, the median time to failure of strategy (TFS) was 16.8 and 9.9 months in the B-E and E-B arms, respectively [hazard ratio (HR)=0.515, 95% CI 0.298-0.889, P=0.017). A similar HR was derived from PSMA for TFS. Using PSMA, TFS was 16.9 and 9.9 months in the B-E and E-B arms, respectively (HR=0.491, 95% CI 0.253-0.952, P=0.031). These results suggested that when both bevacizumab and eribulin are administered, bevacizumab should be administered first and eribulin should be administered later to ensure the most effective use of each drug.
Keywords: bevacizumab; eribulin; sequential treatment.
Copyright: © Matsui et al.
Conflict of interest statement
SM received honoraria from Chugai Pharmaceutical Co., Ltd., and Eisai Co., Ltd., and received research funding (institution) from Eisai Co., Ltd., outside the submitted work.
Figures
References
-
- Cortes J, O'Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, Chollet P, Manikas A, Diéras V, Delozier T, et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open-label randomised study. Lancet. 2011;377:914–923. doi: 10.1016/S0140-6736(11)60070-6. - DOI - PubMed
-
- Robert NJ, Diéras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SYT, Zhou X, et al. RIBBON-1: Randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29:1252–1260. doi: 10.1200/JCO.2010.28.0982. - DOI - PubMed
-
- Miles DW, Chan A, Dirix LY, Cortés J, Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–3247. doi: 10.1200/JCO.2008.21.6457. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous