Degeneracy and stability in neural circuits of dopamine and serotonin neuromodulators: A theoretical consideration
- PMID: 36761394
- PMCID: PMC9905743
- DOI: 10.3389/fncom.2022.950489
Degeneracy and stability in neural circuits of dopamine and serotonin neuromodulators: A theoretical consideration
Abstract
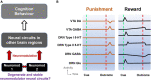
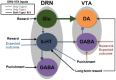
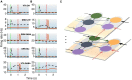
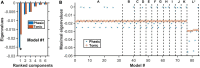
Degenerate neural circuits perform the same function despite being structurally different. However, it is unclear whether neural circuits with interacting neuromodulator sources can themselves degenerate while maintaining the same neuromodulatory function. Here, we address this by computationally modeling the neural circuits of neuromodulators serotonin and dopamine, local glutamatergic and GABAergic interneurons, and their possible interactions, under reward/punishment-based conditioning tasks. The neural modeling is constrained by relevant experimental studies of the VTA or DRN system using, e.g., electrophysiology, optogenetics, and voltammetry. We first show that a single parsimonious, sparsely connected neural circuit model can recapitulate several separate experimental findings that indicated diverse, heterogeneous, distributed, and mixed DRNVTA neuronal signaling in reward and punishment tasks. The inability of this model to recapitulate all observed neuronal signaling suggests potentially multiple circuits acting in parallel. Then using computational simulations and dynamical systems analysis, we demonstrate that several different stable circuit architectures can produce the same observed network activity profile, hence demonstrating degeneracy. Due to the extensive D2-mediated connections in the investigated circuits, we simulate the D2 receptor agonist by increasing the connection strengths emanating from the VTA DA neurons. We found that the simulated D2 agonist can distinguish among sub-groups of the degenerate neural circuits based on substantial deviations in specific neural populations' activities in reward and punishment conditions. This forms a testable model prediction using pharmacological means. Overall, this theoretical work suggests the plausibility of degeneracy within neuromodulator circuitry and has important implications for the stable and robust maintenance of neuromodulatory functions.
Keywords: computational modeling; degeneracy; dopamine; reward and punishment; serotonin.
Copyright © 2023 Behera, Joshi, Wang, Sharp and Wong-Lin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
An integrated modelling framework for neural circuits with multiple neuromodulators.J R Soc Interface. 2017 Jan;14(126):20160902. doi: 10.1098/rsif.2016.0902. J R Soc Interface. 2017. PMID: 28100828 Free PMC article.
-
A network model of basal ganglia for understanding the roles of dopamine and serotonin in reward-punishment-risk based decision making.Front Comput Neurosci. 2015 Jun 17;9:76. doi: 10.3389/fncom.2015.00076. eCollection 2015. Front Comput Neurosci. 2015. PMID: 26136679 Free PMC article.
-
Sensing Positive versus Negative Reward Signals through Adenylyl Cyclase-Coupled GPCRs in Direct and Indirect Pathway Striatal Medium Spiny Neurons.J Neurosci. 2015 Oct 14;35(41):14017-30. doi: 10.1523/JNEUROSCI.0730-15.2015. J Neurosci. 2015. PMID: 26468202 Free PMC article.
-
Modulating the Neuromodulators: Dopamine, Serotonin, and the Endocannabinoid System.Trends Neurosci. 2021 Jun;44(6):464-477. doi: 10.1016/j.tins.2021.02.001. Epub 2021 Mar 3. Trends Neurosci. 2021. PMID: 33674134 Free PMC article. Review.
-
Reward and Aversion.Annu Rev Neurosci. 2016 Jul 8;39:297-324. doi: 10.1146/annurev-neuro-070815-014106. Epub 2016 Apr 21. Annu Rev Neurosci. 2016. PMID: 27145915 Review.
References
LinkOut - more resources
Full Text Sources

