A Cost-Utility Analysis of SQ® Tree SLIT-Tablet versus Placebo in the Treatment of Birch Pollen Allergic Rhinitis from a Swedish Societal Perspective
- PMID: 36761408
- PMCID: PMC9904213
- DOI: 10.2147/CEOR.S377399
A Cost-Utility Analysis of SQ® Tree SLIT-Tablet versus Placebo in the Treatment of Birch Pollen Allergic Rhinitis from a Swedish Societal Perspective
Abstract
Background and aims: Allergic rhinitis (AR) is an immunoglobulin E antibody-mediated inflammatory condition that arises in response to inhaled allergens such as pollen. Pollens from trees in the birch homologous group are the most common allergenic tree pollens in Northern and Central Europe and North America. SQ® Tree SLIT-Tablet (ITULAZAX®) is a sublingual immunotherapy tablet indicated for moderate-to-severe AR and/or conjunctivitis induced by pollen from the birch homologous group. The present analysis evaluated the cost-utility of treating adults with AR with SQ Tree SLIT-Tablet versus placebo, both in combination with symptom-relieving medications, from a Swedish societal perspective.
Methods: A model was developed to evaluate changes in cost and quality of life associated with using SQ Tree SLIT-Tablet relative to placebo in an adult population of individuals with AR. The model captured costs associated with symptom-relieving medications, healthcare professional interactions, SQ Tree SLIT-Tablet, and indirect costs arising from absenteeism and reduced workplace productivity. The analysis was conducted over 10 years with costs captured in 2021 Swedish Krona (SEK) and future costs and effects discounted at 3% per annum. One-way and probabilistic sensitivity analyses were conducted.
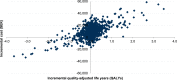
Results: Treatment with SQ Tree SLIT-Tablet resulted in an improvement of 0.041 quality-adjusted life years (QALYs) over 10 years versus placebo. From a Swedish societal perspective, costs increased by SEK 9077 over the same period, resulting in an incremental cost-utility ratio of SEK 223,445 per QALY gained. One-way sensitivity analysis showed that the model was most sensitive to assumptions around the disease-modifying effect of SQ Tree SLIT-Tablet.
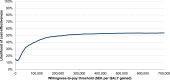
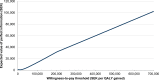
Conclusion: SQ Tree SLIT-Tablet improved quality of life in moderate-to-severe AR and/or conjunctivitis induced by pollen from the birch homologous group in Sweden, with only a modest increase in societal costs over a medium-term time horizon, representing good value for money at a willingness-to-pay threshold of SEK 700,000 per QALY.
Keywords: Sweden; administration; allergic; costs and cost analysis; desensitization; immunologic; oral; quality of life; rhinitis.
© 2023 Pollock et al.
Conflict of interest statement
RFP is a director, shareholder, and full-time employee of Covalence Research Ltd, which received consultancy fees from ALK-Abelló A/S to develop the cost-utility model, formulate and execute the Swedish analyses, and prepare the manuscript draft. AKS, HB, and TSG were full-time employees of ALK-Abelló A/S at the time of the study. The authors report no other conflicts of interest in this work.
Figures

References
-
- European Academy of Allergy and Clinical Immunology (EAACI). Global atlas of allergic rhinitis and chronic rhinosinusitis; 2015.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

