Preparation of β-cyclodextrin/polysaccharide foams using saponin
- PMID: 36761472
- PMCID: PMC9887783
- DOI: 10.3762/bjoc.19.7
Preparation of β-cyclodextrin/polysaccharide foams using saponin
Abstract
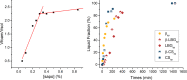
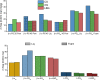
Cyclodextrins, cyclic oligosaccharides with a hydrophobic cavity that form inclusion complexes with nonpolar molecules, can be used to functionalize other polysaccharides. Xanthan gum, locust bean gum or chitosan can be crosslinked using citric acid in the presence of β-cyclodextrin to produce insoluble matrices. In this work, polymeric foams based on those polysaccharides and saponin have been prepared using a green synthesis method to increase the porosity of the matrices. The saponin of soapbark (Quillaja saponaria) has been used to obtain foams using different procedures. The influence of the synthesis path on the porosity of the materials and their corresponding sorption capacities in the aqueous phase were evaluated.
Keywords: chitosan; cyclodextrin polymers; green synthesis; locust bean gum; saponin; sorption; xanthan gum.
Copyright © 2023, Petitjean and Isasi.
Figures






References
LinkOut - more resources
Full Text Sources
