1,4-Dithianes: attractive C2-building blocks for the synthesis of complex molecular architectures
- PMID: 36761474
- PMCID: PMC9907017
- DOI: 10.3762/bjoc.19.12
1,4-Dithianes: attractive C2-building blocks for the synthesis of complex molecular architectures
Abstract
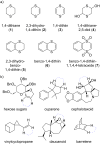
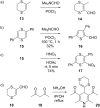
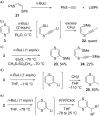
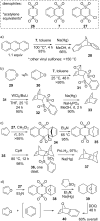
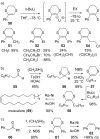
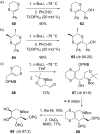
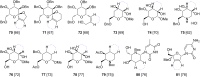
This review covers the synthetic applications of 1,4-dithianes, as well as derivatives thereof at various oxidation states. The selected examples show how the specific heterocyclic reactivity can be harnessed for the controlled synthesis of carbon-carbon bonds. The reactivity is compared to and put into context with more common synthetic building blocks, such as 1,3-dithianes and (hetero)aromatic building blocks. 1,4-Dithianes have as yet not been investigated to the same extent as their well-known 1,3-dithiane counterparts, but they do offer attractive transformations that can find good use in the assembly of a wide array of complex molecular architectures, ranging from lipids and carbohydrates to various carbocyclic scaffolds. This versatility arises from the possibility to chemoselectively cleave or reduce the sulfur-heterocycle to reveal a versatile C2-synthon.
Keywords: 1,4-dithianes; 1,4-dithiins; 2,3-dihydro-1,4-dithiins; heterocycles; target synthesis.
Copyright © 2023, Ryckaert et al.
Figures


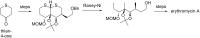








Similar articles
-
Reactions of 2-Aryl-1,3-Dithianes and [1.1.1]Propellane.Angew Chem Int Ed Engl. 2019 Sep 16;58(38):13416-13420. doi: 10.1002/anie.201905531. Epub 2019 Aug 7. Angew Chem Int Ed Engl. 2019. PMID: 31291500 Free PMC article.
-
Enantioselective Addition of a 2-Alkoxycarbonyl-1,3-dithiane to Imines Catalyzed by a Bis(guanidino)iminophosphorane Organosuperbase.Angew Chem Int Ed Engl. 2015 Dec 21;54(52):15836-9. doi: 10.1002/anie.201508178. Epub 2015 Oct 20. Angew Chem Int Ed Engl. 2015. PMID: 26480953
-
Trifluoromethyl-β-dicarbonyls as Versatile Synthons in Synthesis of Heterocycles.Chemistry. 2024 Feb 21;30(11):e202303599. doi: 10.1002/chem.202303599. Epub 2024 Jan 4. Chemistry. 2024. PMID: 38055226 Review.
-
Synthesis of isotopically labeled 1,3-dithiane.J Labelled Comp Radiopharm. 2014 May 15;57(5):338-41. doi: 10.1002/jlcr.3185. Epub 2014 Feb 7. J Labelled Comp Radiopharm. 2014. PMID: 24861982
-
Alkynoates as Versatile and Powerful Chemical Tools for the Rapid Assembly of Diverse Heterocycles under Transition-Metal Catalysis: Recent Developments and Challenges.Top Curr Chem (Cham). 2021 Jan 5;379(1):3. doi: 10.1007/s41061-020-00316-4. Top Curr Chem (Cham). 2021. PMID: 33398642 Review.
Cited by
-
Organic Peroxides in Transition-Metal-Free Cyclization and Coupling Reactions (C-C) via Oxidative Transformation.ACS Omega. 2025 Apr 14;10(16):15852-15907. doi: 10.1021/acsomega.4c11574. eCollection 2025 Apr 29. ACS Omega. 2025. PMID: 40321530 Free PMC article. Review.
References
-
- Corey E J, Seebach D. Angew Chem, Int Ed Engl. 1965;4:1075–1077. doi: 10.1002/anie.196510752. - DOI
-
- Seebach D, Corey E J. J Org Chem. 1975;40:231–237. doi: 10.1021/jo00890a018. - DOI
-
- Seebach D. Angew Chem, Int Ed Engl. 1979;18:239–258. doi: 10.1002/anie.197902393. - DOI
-
- Yus M, Nájera C, Foubelo F. Tetrahedron. 2003;59:6147–6212. doi: 10.1016/s0040-4020(03)00955-4. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
