Stem cell- derived extracellular vesicles as new tools in regenerative medicine - Immunomodulatory role and future perspectives
- PMID: 36761725
- PMCID: PMC9902918
- DOI: 10.3389/fimmu.2023.1120175
Stem cell- derived extracellular vesicles as new tools in regenerative medicine - Immunomodulatory role and future perspectives
Abstract
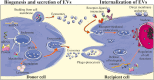
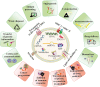
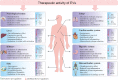
In the last few decades, the practical use of stem cells (SCs) in the clinic has attracted significant attention in the regenerative medicine due to the ability of these cells to proliferate and differentiate into other cell types. However, recent findings have demonstrated that the therapeutic capacity of SCs may also be mediated by their ability to secrete biologically active factors, including extracellular vesicles (EVs). Such submicron circular membrane-enveloped vesicles may be released from the cell surface and harbour bioactive cargo in the form of proteins, lipids, mRNA, miRNA, and other regulatory factors. Notably, growing evidence has indicated that EVs may transfer their bioactive content into recipient cells and greatly modulate their functional fate. Thus, they have been recently envisioned as a new class of paracrine factors in cell-to-cell communication. Importantly, EVs may modulate the activity of immune system, playing an important role in the regulation of inflammation, exhibiting broad spectrum of the immunomodulatory activity that promotes the transition from pro-inflammatory to pro-regenerative environment in the site of tissue injury. Consequently, growing interest is placed on attempts to utilize EVs in clinical applications of inflammatory-related dysfunctions as potential next-generation therapeutic factors, alternative to cell-based approaches. In this review we will discuss the current knowledge on the biological properties of SC-derived EVs, with special focus on their role in the regulation of inflammatory response. We will also address recent findings on the immunomodulatory and pro-regenerative activity of EVs in several disease models, including in vitro and in vivo preclinical, as well as clinical studies. Finally, we will highlight the current perspectives and future challenges of emerging EV-based therapeutic strategies of inflammation-related diseases treatment.
Keywords: extracellular vesicles; immunomodulation; inflammation; paracrine activity; regenerative medicine; stem cells; tissue injury.
Copyright © 2023 Karnas, Dudek and Zuba-Surma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Stem Cells-Derived Extracellular Vesicles: Potential Therapeutics for Wound Healing in Chronic Inflammatory Skin Diseases.Int J Mol Sci. 2021 Mar 19;22(6):3130. doi: 10.3390/ijms22063130. Int J Mol Sci. 2021. PMID: 33808520 Free PMC article. Review.
-
Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles: A focus on inflammatory bowel disease.Clin Transl Med. 2024 Nov;14(11):e70075. doi: 10.1002/ctm2.70075. Clin Transl Med. 2024. PMID: 39488745 Free PMC article. Review.
-
Therapeutic Advances of Stem Cell-Derived Extracellular Vesicles in Regenerative Medicine.Cells. 2020 Mar 13;9(3):707. doi: 10.3390/cells9030707. Cells. 2020. PMID: 32183102 Free PMC article. Review.
-
Mesenchymal Stem Cell-Derived Extracellular Vesicles in Tissue Regeneration.Cell Transplant. 2020 Jan-Dec;29:963689720908500. doi: 10.1177/0963689720908500. Cell Transplant. 2020. PMID: 32207341 Free PMC article. Review.
-
Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis.Cell Tissue Res. 2018 Oct;374(1):1-15. doi: 10.1007/s00441-018-2871-5. Epub 2018 Jun 28. Cell Tissue Res. 2018. PMID: 29955951 Review.
Cited by
-
Embryotrophic effect of exogenous protein contained adipose-derived stem cell extracellular vesicles.J Anim Sci Biotechnol. 2024 Nov 3;15(1):145. doi: 10.1186/s40104-024-01106-4. J Anim Sci Biotechnol. 2024. PMID: 39488683 Free PMC article.
-
Lipid Metabolism Modulation during SARS-CoV-2 Infection: A Spotlight on Extracellular Vesicles and Therapeutic Prospects.Int J Mol Sci. 2024 Jan 4;25(1):640. doi: 10.3390/ijms25010640. Int J Mol Sci. 2024. PMID: 38203811 Free PMC article. Review.
-
Extracellular RNA communication: A decade of NIH common fund support illuminates exRNA biology.J Extracell Vesicles. 2025 Jan;14(1):e70016. doi: 10.1002/jev2.70016. J Extracell Vesicles. 2025. PMID: 39815775 Free PMC article. Review.
-
Extracellular Vesicles in cancer: from isolation and characterization to metastasis, drug resistance, and clinical applications.BMC Cancer. 2025 Jul 8;25(1):1154. doi: 10.1186/s12885-025-14375-7. BMC Cancer. 2025. PMID: 40629268 Free PMC article. Review.
-
A Paradigm Shift in SSTI Management: The Multifunctional Role of Extracellular Vesicles.Int J Mol Sci. 2025 Jul 5;26(13):6481. doi: 10.3390/ijms26136481. Int J Mol Sci. 2025. PMID: 40650257 Free PMC article. Review.
References
-
- Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, et al. . Minimal information for studies of extracellular vesicles 2018 (Misev2018): A position statement of the international society for extracellular vesicles and update of the Misev2014 guidelines. J Extracell Vesicles (2018) 7(1):1535750. doi: 10.1080/20013078.2018.1535750 - DOI - PMC - PubMed
-
- Foster BP, Balassa T, Benen TD, Dominovic M, Elmadjian GK, Florova V, et al. . Extracellular vesicles in blood, milk and body fluids of the female and Male urogenital tract and with special regard to reproduction. Crit Rev Clin Lab Sci (2016) 53(6):379–95. doi: 10.1080/10408363.2016.1190682 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

