Silent neonatal influenza A virus infection primes systemic antimicrobial immunity
- PMID: 36761727
- PMCID: PMC9902881
- DOI: 10.3389/fimmu.2023.1072142
Silent neonatal influenza A virus infection primes systemic antimicrobial immunity
Abstract
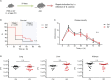
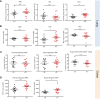
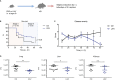
Infections with influenza A viruses (IAV) cause seasonal epidemics and global pandemics. The majority of these infections remain asymptomatic, especially among children below five years of age. Importantly, this is a time, when immunological imprinting takes place. Whether early-life infections with IAV affect the development of antimicrobial immunity is unknown. Using a preclinical mouse model, we demonstrate here that silent neonatal influenza infections have a remote beneficial impact on the later control of systemic juvenile-onset and adult-onset infections with an unrelated pathogen, Staphylococcus aureus, due to improved pathogen clearance and clinical resolution. Strategic vaccination with a live attenuated IAV vaccine elicited a similar protection phenotype. Mechanistically, the IAV priming effect primarily targets antimicrobial functions of the developing innate immune system including increased antimicrobial plasma activity and enhanced phagocyte functions and antigen-presenting properties at mucosal sites. Our results suggest a long-term benefit from an exposure to IAV during the neonatal phase, which might be exploited by strategic vaccination against influenza early in life to enforce the host's resistance to later bacterial infections.
Keywords: Staphylococcus aureus; antimicrobial immunity; influenza A virus; influenza vaccination; innate immunity training; neonate; sepsis.
Copyright © 2023 Heinemann, Stalp, Bonifacio, Silva, Willers, Heckmann, Fehlhaber, Völlger, Raafat, Normann, Klos, Hansen, Schmolke and Viemann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Modulation of Innate Immune Responses by the Influenza A NS1 and PA-X Proteins.Viruses. 2018 Dec 12;10(12):708. doi: 10.3390/v10120708. Viruses. 2018. PMID: 30545063 Free PMC article. Review.
-
Inactivated Influenza Vaccine That Provides Rapid, Innate-Immune-System-Mediated Protection and Subsequent Long-Term Adaptive Immunity.mBio. 2015 Oct 27;6(6):e01024-15. doi: 10.1128/mBio.01024-15. mBio. 2015. PMID: 26507227 Free PMC article.
-
Development of an Alternative Modified Live Influenza B Virus Vaccine.J Virol. 2017 May 26;91(12):e00056-17. doi: 10.1128/JVI.00056-17. Print 2017 Jun 15. J Virol. 2017. PMID: 28381580 Free PMC article.
-
CCR2 Regulates Vaccine-Induced Mucosal T-Cell Memory to Influenza A Virus.J Virol. 2021 Jul 12;95(15):e0053021. doi: 10.1128/JVI.00530-21. Epub 2021 Jul 12. J Virol. 2021. PMID: 33952647 Free PMC article.
-
Innovative Mucosal Vaccine Formulations Against Influenza A Virus Infections.Front Immunol. 2019 Jul 17;10:1605. doi: 10.3389/fimmu.2019.01605. eCollection 2019. Front Immunol. 2019. PMID: 31379823 Free PMC article. Review.
Cited by
-
Influenza Immunization in Very-Low-Birth-Weight Infants: Epidemiology and Long-Term Outcomes.Vaccines (Basel). 2025 Jan 7;13(1):42. doi: 10.3390/vaccines13010042. Vaccines (Basel). 2025. PMID: 39852821 Free PMC article.
References
-
- Thompson MG, Levine MZ, Bino S, Hunt DR, Al-Sanouri TM, Simões EAF, et al. . Underdetection of laboratory-confirmed influenza-associated hospital admissions among infants: A multicentre, prospective study. Lancet Child Adolesc Health (2019) 3:781–94. doi: 10.1016/S2352-4642(19)30246-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

