Platelets derived citrullinated proteins and microparticles are potential autoantibodies ACPA targets in RA patients
- PMID: 36761728
- PMCID: PMC9902922
- DOI: 10.3389/fimmu.2023.1084283
Platelets derived citrullinated proteins and microparticles are potential autoantibodies ACPA targets in RA patients
Abstract
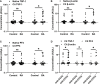
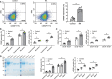
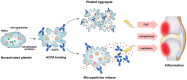
Citrullinated neoepitopes have emerged as key triggers of autoantibodies anti-citrullinated protein antibodies (ACPA) synthesis in rheumatoid arthritis (RA) patients. Apart from their critical role in homeostasis and thrombosis, platelets have a significant contribution to inflammation as well. Although anuclear in nature, platelets have an intricate post-translational modification machinery. Till now, citrullination in platelets and its contribution to trigger autoantibodies ACPA production in RA is an unexplored research direction. Herein, we investigated the expression of peptidylarginine deiminase (PAD) enzymes and citrullinated proteins/peptides in the human platelets and platelet derived microparticles (PDP). Both PAD4 mRNA and protein, but not the other PAD isoforms, are detectable in the human platelets. With a strict filtering criterion,108 citrullination sites present on 76 proteins were identified in the human platelets, and 55 citrullinated modifications present on 37 different proteins were detected in the PDPs. Among them, some are well-known citrullinated autoantigens associated with RA. Citrullinated forms of thrombospondin-1, β-actin, and platelet factor-4 (also known as CXCL4) are highly immunogenic and bound by autoantibodies ACPA. Furthermore, ACPA from RA sera and synovial fluids recognized citrullinated proteins from platelets and significantly activated them as evidenced by P-selectin upregulation and sCD40 L secretion. These results clearly demonstrate the presence of citrullinated autoantigens in platelets and PDPs, thus could serve as potential targets of ACPA in RA.
Keywords: anti-citrullinated protein antibodies (ACPA); citrullination; platelet derived microparticles (PDP); platelets; rheumatoid arthritis.
Copyright © 2023 Xu, Du, Xing, Chen, Wan, Wang, Xiong, Nandakumar, Holmdahl and Geng.
Conflict of interest statement
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer SC declared a shared parent affiliation with the author RD at the time of review.
Figures


Similar articles
-
Identification of potential autoantigens in anti-CCP-positive and anti-CCP-negative rheumatoid arthritis using citrulline-specific protein arrays.Sci Rep. 2021 Aug 27;11(1):17300. doi: 10.1038/s41598-021-96675-z. Sci Rep. 2021. PMID: 34453079 Free PMC article.
-
Differential ACPA Binding to Nuclear Antigens Reveals a PAD-Independent Pathway and a Distinct Subset of Acetylation Cross-Reactive Autoantibodies in Rheumatoid Arthritis.Front Immunol. 2019 Jan 4;9:3033. doi: 10.3389/fimmu.2018.03033. eCollection 2018. Front Immunol. 2019. PMID: 30662440 Free PMC article.
-
Citrullinated Autoantigens: From Diagnostic Markers to Pathogenetic Mechanisms.Clin Rev Allergy Immunol. 2015 Oct;49(2):232-9. doi: 10.1007/s12016-014-8459-2. Clin Rev Allergy Immunol. 2015. PMID: 25355199 Review.
-
Characterization of Autoantigens Targeted by Anti-Citrullinated Protein Antibodies In Vivo: Prominent Role for Epitopes Derived from Histone 4 Proteins.PLoS One. 2016 Oct 27;11(10):e0165501. doi: 10.1371/journal.pone.0165501. eCollection 2016. PLoS One. 2016. PMID: 27788229 Free PMC article.
-
Periodontal sources of citrullinated antigens and TLR agonists related to RA.Autoimmunity. 2018 Sep;51(6):304-309. doi: 10.1080/08916934.2018.1527907. Epub 2018 Nov 10. Autoimmunity. 2018. PMID: 30417696 Review.
Cited by
-
Platelet-Derived Microparticles and Autoimmune Diseases.Int J Mol Sci. 2023 Jun 17;24(12):10275. doi: 10.3390/ijms241210275. Int J Mol Sci. 2023. PMID: 37373420 Free PMC article. Review.
-
Aberrant Activation of Immune and Non-Immune Cells Contributes to Joint Inflammation and Bone Degradation in Rheumatoid Arthritis.Int J Mol Sci. 2023 Nov 1;24(21):15883. doi: 10.3390/ijms242115883. Int J Mol Sci. 2023. PMID: 37958864 Free PMC article. Review.
-
Platelet-Derived Soluble CD40L and Its Impact on Immune Modulation and Anti-IL6R Antibody Treatment Outcome in Rheumatoid Arthritis.Cells. 2025 Apr 22;14(9):625. doi: 10.3390/cells14090625. Cells. 2025. PMID: 40358149 Free PMC article.
-
The Role of Autophagy as a Trigger of Post-Translational Modifications of Proteins and Extracellular Vesicles in the Pathogenesis of Rheumatoid Arthritis.Int J Mol Sci. 2023 Aug 14;24(16):12764. doi: 10.3390/ijms241612764. Int J Mol Sci. 2023. PMID: 37628944 Free PMC article. Review.
-
GnRH Induces Citrullination of the Cytoskeleton in Murine Gonadotrope Cells.Int J Mol Sci. 2024 Mar 10;25(6):3181. doi: 10.3390/ijms25063181. Int J Mol Sci. 2024. PMID: 38542155 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

