The altering cellular components and function in tumor microenvironment during remissive and relapsed stages of anti-CD19 CAR T-cell treated lymphoma mice
- PMID: 36761733
- PMCID: PMC9905118
- DOI: 10.3389/fimmu.2023.1101769
The altering cellular components and function in tumor microenvironment during remissive and relapsed stages of anti-CD19 CAR T-cell treated lymphoma mice
Abstract
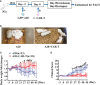
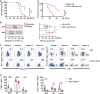
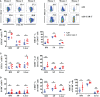
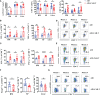
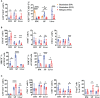
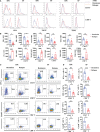
Anti-CD19 chimeric antigen receptor (CAR) T cells represent a highly promising strategy for B-cell malignancies. Despite the inspiring initial achievement, remission in a notable fraction of subjects is short-lived, and relapse remains a major challenge. Tumor microenvironment (TME) was proved to be aroused by CAR T cells; however, little is known about the dynamic characteristics of cellular components in TME especially during the different phases of disease after anti-CD19 CAR T-cell treatment. We took advantage of an immunocompetent model receiving syngeneic A20 lymphoma cells to dissect the changes in TME with or without CAR T-cell injection. We found that anti-CD19 CAR T-cell treatment attenuated the symptoms of lymphoma and significantly prolonged mice survival through eradicating systemic CD19+ cells. Increased myeloid subsets, including CD11c+ DCs and F4/80+ macrophages with higher MHC II and CD80 expression in bone marrow, spleen, and liver, were detected when mice reached remission after anti-CD19 CAR T treatment. Compared to mice without anti-CD19 CAR T administration, intrinsic T cells were triggered to produce more IFN-γ and TNF-α. However, some lymphoma mice relapsed by day 42 after therapy, which coincided with CAR T-cell recession, decreased myeloid cell activation and increased Treg cells. Elevated intrinsic T cells with high PD-1 and TIGIT exhaust signatures and attenuated cytotoxicity in TME were associated with the late-stage relapse of CAR T-cell treatment. In summary, the cellular compositions of TME as allies of CAR T cells may contribute to the anti-tumor efficacy at the initial stage, whereas anti-CD19 CAR T-cell disappearance and host response immunosuppression may work together to cause lymphoma relapse after an initial, near-complete elimination phase.
Keywords: CD19; chimeric antigen receptor T cell; immunosuppression; lymphoma; tumor microenvironment.
Copyright © 2023 Zhao, Ren, Tang, Zhao, Chen, Wang and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The transition between M1 and M2 macrophage phenotypes is associated with the disease status following CD19 CAR-T therapy for B cell lymphoma/leukemia.Cell Death Dis. 2025 Apr 11;16(1):275. doi: 10.1038/s41419-025-07610-3. Cell Death Dis. 2025. PMID: 40216772 Free PMC article.
-
Expression of VISTA regulated via IFN-γ governs endogenous T-cell function and exhibits correlation with the efficacy of CD19 CAR-T cell treated B-malignant mice.J Immunother Cancer. 2024 Jun 25;12(6):e008364. doi: 10.1136/jitc-2023-008364. J Immunother Cancer. 2024. PMID: 38925679 Free PMC article.
-
Clinical Efficacy and Tumor Microenvironment Influence in a Dose-Escalation Study of Anti-CD19 Chimeric Antigen Receptor T Cells in Refractory B-Cell Non-Hodgkin's Lymphoma.Clin Cancer Res. 2019 Dec 1;25(23):6995-7003. doi: 10.1158/1078-0432.CCR-19-0101. Epub 2019 Aug 23. Clin Cancer Res. 2019. PMID: 31444250 Clinical Trial.
-
Secondary myeloid neoplasms after CD19 CAR T therapy in patients with refractory/relapsed B-cell lymphoma: Case series and review of literature.Front Immunol. 2023 Jan 13;13:1063986. doi: 10.3389/fimmu.2022.1063986. eCollection 2022. Front Immunol. 2023. PMID: 36713414 Free PMC article. Review.
-
Multispecific CAR T Cells Deprive Lymphomas of Escape via Antigen Loss.Annu Rev Med. 2023 Jan 27;74:279-291. doi: 10.1146/annurev-med-042921-024719. Epub 2022 Nov 4. Annu Rev Med. 2023. PMID: 36332638 Review.
Cited by
-
The transition between M1 and M2 macrophage phenotypes is associated with the disease status following CD19 CAR-T therapy for B cell lymphoma/leukemia.Cell Death Dis. 2025 Apr 11;16(1):275. doi: 10.1038/s41419-025-07610-3. Cell Death Dis. 2025. PMID: 40216772 Free PMC article.
-
Co-inhibition of TIGIT and PD-1/PD-L1 in Cancer Immunotherapy: Mechanisms and Clinical Trials.Mol Cancer. 2023 Jun 8;22(1):93. doi: 10.1186/s12943-023-01800-3. Mol Cancer. 2023. PMID: 37291608 Free PMC article. Review.
-
The Bidirectional Interplay between T Cell-Based Immunotherapies and the Tumor Microenvironment.Cancer Immunol Res. 2025 Apr 2;13(4):463-475. doi: 10.1158/2326-6066.CIR-24-0857. Cancer Immunol Res. 2025. PMID: 39786986 Free PMC article. Review.
-
In vivo manufacture and manipulation of CAR-T cells for better druggability.Cancer Metastasis Rev. 2024 Sep;43(3):1075-1093. doi: 10.1007/s10555-024-10185-8. Epub 2024 Apr 9. Cancer Metastasis Rev. 2024. PMID: 38592427 Review.
-
Expression of VISTA regulated via IFN-γ governs endogenous T-cell function and exhibits correlation with the efficacy of CD19 CAR-T cell treated B-malignant mice.J Immunother Cancer. 2024 Jun 25;12(6):e008364. doi: 10.1136/jitc-2023-008364. J Immunother Cancer. 2024. PMID: 38925679 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

