Specific myeloid signatures in peripheral blood differentiate active and rare clinical phenotypes of multiple sclerosis
- PMID: 36761741
- PMCID: PMC9905713
- DOI: 10.3389/fimmu.2023.1071623
Specific myeloid signatures in peripheral blood differentiate active and rare clinical phenotypes of multiple sclerosis
Abstract
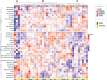
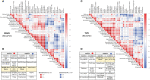
Current understanding of Multiple Sclerosis (MS) pathophysiology implicates perturbations in adaptive cellular immune responses, predominantly T cells, in Relapsing-Remitting forms (RRMS). Nevertheless, from a clinical perspective MS is a heterogeneous disease reflecting the heterogeneity of involved biological systems. This complexity requires advanced analysis tools at the single-cell level to discover biomarkers for better patient-group stratification. We designed a novel 44-parameter mass cytometry panel to interrogate predominantly the role of effector and regulatory subpopulations of peripheral blood myeloid subsets along with B and T-cells (excluding granulocytes) in MS, assessing three different patient cohorts: RRMS, PPMS (Primary Progressive) and Tumefactive MS patients (TMS) (n=10, 8, 14 respectively). We further subgrouped our cohort into inactive or active disease stages to capture the early underlying events in disease pathophysiology. Peripheral blood analysis showed that TMS cases belonged to the spectrum of RRMS, whereas PPMS cases displayed different features. In particular, TMS patients during a relapse stage were characterized by a specific subset of CD11c+CD14+ CD33+, CD192+, CD172+-myeloid cells with an alternative phenotype of monocyte-derived macrophages (high arginase-1, CD38, HLA-DR-low and endogenous TNF-a production). Moreover, TMS patients in relapse displayed a selective CD4 T-cell lymphopenia of cells with a Th2-like polarised phenotype. PPMS patients did not display substantial differences from healthy controls, apart from a trend toward higher expansion of NK cell subsets. Importantly, we found that myeloid cell populations are reshaped under effective disease-modifying therapy predominantly with glatiramer acetate and to a lesser extent with anti-CD20, suggesting that the identified cell signature represents a specific therapeutic target in TMS. The expanded myeloid signature in TMS patients was also confirmed by flow cytometry. Serum neurofilament light-chain levels confirmed the correlation of this myeloid cell signature with indices of axonal injury. More in-depth analysis of myeloid subsets revealed an increase of a subset of highly cytolytic and terminally differentiated NK cells in PPMS patients with leptomeningeal enhancement (active-PPMS), compared to those without (inactive-PPMS). We have identified previously uncharacterized subsets of circulating myeloid cells and shown them to correlate with distinct disease forms of MS as well as with specific disease states (relapse/remission).
Keywords: B cells; NK cells; T cells; macrophages; mass cytometry; multiple sclerosis; myeloid-signature; tumefactive multiple sclerosis.
Copyright © 2023 Vakrakou, Paschalidis, Pavlos, Giannouli, Karathanasis, Tsipota, Velonakis, Stadelmann-Nessler, Evangelopoulos, Stefanis and Kilidireas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Multiple sclerosis by phenotype in Germany.Mult Scler Relat Disord. 2022 Jan;57:103326. doi: 10.1016/j.msard.2021.103326. Epub 2021 Oct 10. Mult Scler Relat Disord. 2022. PMID: 35158442
-
Specific alterations in NKG2D+ T lymphocytes in relapsing-remitting and progressive multiple sclerosis patients.Mult Scler Relat Disord. 2023 Mar;71:104542. doi: 10.1016/j.msard.2023.104542. Epub 2023 Jan 26. Mult Scler Relat Disord. 2023. PMID: 36716577
-
Phenotypic and functional alterations of myeloid-derived suppressor cells during the disease course of multiple sclerosis.Immunol Cell Biol. 2018 Sep;96(8):820-830. doi: 10.1111/imcb.12042. Epub 2018 Apr 19. Immunol Cell Biol. 2018. PMID: 29569304
-
Mitoxantrone: a review of its use in multiple sclerosis.CNS Drugs. 2004;18(6):379-96. doi: 10.2165/00023210-200418060-00010. CNS Drugs. 2004. PMID: 15089110 Review.
-
Gray Matter Atrophy in the Cortico-Striatal-Thalamic Network and Sensorimotor Network in Relapsing-Remitting and Primary Progressive Multiple Sclerosis.Neuropsychol Rev. 2021 Dec;31(4):703-720. doi: 10.1007/s11065-021-09479-3. Epub 2021 Feb 13. Neuropsychol Rev. 2021. PMID: 33582965 Review.
Cited by
-
Identification of shared pathogenic signatures of multiple sclerosis and chronic obstructive pulmonary disease: an integrated transcriptomic analysis of blood specimens.Mol Genet Genomics. 2024 Dec 27;300(1):8. doi: 10.1007/s00438-024-02215-5. Mol Genet Genomics. 2024. PMID: 39725779
-
Increased interferon I signaling, DNA damage response and evidence of T-cell exhaustion in a patient with combined interferonopathy (Aicardi-Goutières Syndrome, AGS) and cohesinopathy (Cornelia de Lange Syndrome, CdLS).Pediatr Rheumatol Online J. 2025 Jan 27;23(1):11. doi: 10.1186/s12969-024-01050-7. Pediatr Rheumatol Online J. 2025. PMID: 39871364 Free PMC article.
-
Multiparametric analysis in the peripheral blood of Giant Cell Arteritis and Polymyalgia Rheumatica patients at the early phases of steroid treatment reveals changes in cell subpopulations and lipid mediators: a preliminary study.Front Immunol. 2025 Jun 4;16:1594263. doi: 10.3389/fimmu.2025.1594263. eCollection 2025. Front Immunol. 2025. PMID: 40534888 Free PMC article.
-
Neurodegeneration correlates of iron-related lesions and leptomeningeal inflammation in multiple sclerosis clinical subtypes.Neuroradiology. 2025 Jun;67(6):1541-1555. doi: 10.1007/s00234-025-03595-0. Epub 2025 Mar 25. Neuroradiology. 2025. PMID: 40131429 Free PMC article.
-
Single-Cell Analysis of Bone Marrow CD8+ T Cells in Myeloid Neoplasms Reveals Pathways Associated with Disease Progression and Response to Treatment with Azacitidine.Cancer Res Commun. 2024 Dec 1;4(12):3067-3083. doi: 10.1158/2767-9764.CRC-24-0310. Cancer Res Commun. 2024. PMID: 39485042 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

