Analysis of causes for poor persistence of CAR-T cell therapy in vivo
- PMID: 36761742
- PMCID: PMC9905114
- DOI: 10.3389/fimmu.2023.1063454
Analysis of causes for poor persistence of CAR-T cell therapy in vivo
Abstract
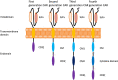
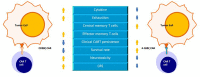
Chimeric antigen receptor T-cell (CAR-T-cell) therapy has been well researched to date because of its ability to target malignant tumor cells. The most common CAR-T cells are CD19 CAR-T cells, which play a large role in B-cell leukemia treatment. However, most CAR-T cells are associated with relapse after clinical treatment, so the quality and persistence of CAR-T cells need to be improved. With continuous optimization, there have been four generations of CARs and each generation of CARs has better quality and durability than the previous generation. In addition, it is important to increase the proportion of memory cells in CAR-T cells. Studies have shown that an immunosuppressive tumor microenvironment (TME) can lead to dysfunction of CAR-T cells, resulting in decreased cell proliferation and poor persistence. Thus, overcoming the challenges of immunosuppressive molecules and targeting cytokines in the TME can also improve CAR-T cell persistence. In this paper, we explored how to improve the durability of CAR-T cell therapy by improving the structure of CARs, increasing the proportion of memory CAR-T cells and improving the TME.
Keywords: CAR-T cells; relapsed/refractory; the proportion of memory CAR-T cells; the structure of CARs; tumor microenvironment.
Copyright © 2023 Kong, Tang, You, Li and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Chimeric Antigen Receptor T Cell Therapy for Pediatric B-ALL: Narrowing the Gap Between Early and Long-Term Outcomes.Front Immunol. 2020 Aug 11;11:1985. doi: 10.3389/fimmu.2020.01985. eCollection 2020. Front Immunol. 2020. PMID: 32849662 Free PMC article. Review.
-
Chimeric antigen receptor T-cell lymphoma immunotherapy: the next questions.Curr Opin Oncol. 2020 Sep;32(5):434-441. doi: 10.1097/CCO.0000000000000671. Curr Opin Oncol. 2020. PMID: 32796231 Review.
-
Chimeric Antigen Receptor T Cell Therapy for Acute Lymphoblastic Leukemia.Curr Treat Options Oncol. 2020 Feb 5;21(2):16. doi: 10.1007/s11864-020-0706-6. Curr Treat Options Oncol. 2020. PMID: 32025828 Review.
-
CAR T Cells for Solid Tumors: New Strategies for Finding, Infiltrating, and Surviving in the Tumor Microenvironment.Front Immunol. 2019 Feb 5;10:128. doi: 10.3389/fimmu.2019.00128. eCollection 2019. Front Immunol. 2019. PMID: 30804938 Free PMC article. Review.
-
A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines.J Immunother Cancer. 2017 May 16;5:42. doi: 10.1186/s40425-017-0246-1. eCollection 2017. J Immunother Cancer. 2017. PMID: 28515942 Free PMC article.
Cited by
-
Strategies for Reducing Toxicity and Enhancing Efficacy of Chimeric Antigen Receptor T Cell Therapy in Hematological Malignancies.Int J Mol Sci. 2023 May 23;24(11):9115. doi: 10.3390/ijms24119115. Int J Mol Sci. 2023. PMID: 37298069 Free PMC article. Review.
-
An overview on in-vivo generation of CAR-T cells using CRISPR-loaded functionalized nanocarriers for treating B-cell lineage acute lymphoblastic leukemia.Mol Biol Rep. 2025 Jun 14;52(1):596. doi: 10.1007/s11033-025-10674-1. Mol Biol Rep. 2025. PMID: 40515942 Review.
-
Emerging Strategies to Overcome Current CAR-T Therapy Dilemmas - Exosomes Derived from CAR-T Cells.Int J Nanomedicine. 2024 Mar 18;19:2773-2791. doi: 10.2147/IJN.S445101. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38525009 Free PMC article. Review.
-
Outcomes of Idecabtagene Vicleucel Therapy in Patients with Relapsed/Refractory Multiple Myeloma: A Single-Institution Experience.Biomedicines. 2024 Dec 27;13(1):36. doi: 10.3390/biomedicines13010036. Biomedicines. 2024. PMID: 39857619 Free PMC article.
-
Strategies for Altering Delivery Technologies to Optimize CAR Therapy.Int J Mol Sci. 2025 Mar 30;26(7):3206. doi: 10.3390/ijms26073206. Int J Mol Sci. 2025. PMID: 40244018 Free PMC article. Review.
References
-
- Heuser C, Hombach A, Losch C, Manista K, Abken H. T-Cell activation by recombinant immunoreceptors: Impact of the intracellular signalling domain on the stability of receptor expression and antigen-specific activation of grafted T cells. Gene Ther (2003) 10(17):1408–19. doi: 10.1038/sj.gt.3302023 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

