T cell immunity ameliorates COVID-19 disease severity and provides post-exposure prophylaxis after peptide-vaccination, in Syrian hamsters
- PMID: 36761759
- PMCID: PMC9902696
- DOI: 10.3389/fimmu.2023.1111629
T cell immunity ameliorates COVID-19 disease severity and provides post-exposure prophylaxis after peptide-vaccination, in Syrian hamsters
Abstract
Background: The emergence of novel SARS-CoV-2 variants that resist neutralizing antibodies drew the attention to cellular immunity and calls for the development of alternative vaccination strategies to combat the pandemic. Here, we have assessed the kinetics of T cell responses and protective efficacy against severe COVID-19 in pre- and post-exposure settings, elicited by PolyPEPI-SCoV-2, a peptide based T cell vaccine.
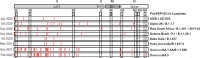
Methods: 75 Syrian hamsters were immunized subcutaneously with PolyPEPI-SCoV-2 on D0 and D14. On D42, hamsters were intranasally challenged with 102 TCID50 of the virus. To analyze immunogenicity by IFN-γ ELISPOT and antibody secretion, lymphoid tissues were collected both before (D0, D14, D28, D42) and after challenge (D44, D46, D49). To measure vaccine efficacy, lung tissue, throat swabs and nasal turbinate samples were assessed for viral load and histopathological changes. Further, body weight was monitored on D0, D28, D42 and every day after challenge.
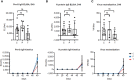
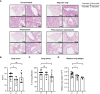
Results: The vaccine induced robust activation of T cells against all SARS-CoV-2 structural proteins that were rapidly boosted after virus challenge compared to control animals (~4-fold, p<0.05). A single dose of PolyPEPI-SCoV-2 administered one day after challenge also resulted in elevated T cell response (p<0.01). The vaccination did not induce virus-specific antibodies and viral load reduction. Still, peptide vaccination significantly reduced body weight loss (p<0.001), relative lung weight (p<0.05) and lung lesions (p<0.05), in both settings.
Conclusion: Our study provides first proof of concept data on the contribution of T cell immunity on disease course and provide rationale for the use of T cell-based peptide vaccines against both novel SARS-CoV-2 variants and supports post-exposure prophylaxis as alternative vaccination strategy against COVID-19.
Keywords: SARS-CoV-2; T cells; adaptive immunity; post-exposure prophylaxis; therapeutic; vaccine.
Copyright © 2023 Somogyi, Kremlitzka, Csiszovszki, Molnár, Lőrincz, Tóth, de Waal, Pattijn, Reineking, Beineke and Tőke.
Conflict of interest statement
ET, ES, MK, JT, LM, ZC, and OL are employees of Treos Bio Zrt. and ET, ES, JT, LM, ZC, OL hold shares in Treos Bio Ltd. and are listed as inventors of the following patents: US10973909B1 and PCT/GB2021/050829. SP is employed by ImmunXperts SA, a Nexelis company, LW is employee of Viroclinics Biosciences B.V. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Absence of Vaccine-enhanced Disease With Unexpected Positive Protection Against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by Inactivated Vaccine Given Within 3 Days of Virus Challenge in Syrian Hamster Model.Clin Infect Dis. 2021 Aug 2;73(3):e719-e734. doi: 10.1093/cid/ciab083. Clin Infect Dis. 2021. PMID: 33515458 Free PMC article.
-
Prototype and BA.5 protein nanoparticle vaccines protect against Omicron BA.5 variant in Syrian hamsters.J Virol. 2024 Mar 19;98(3):e0120623. doi: 10.1128/jvi.01206-23. Epub 2024 Feb 2. J Virol. 2024. PMID: 38305154 Free PMC article.
-
An Intranasal OMV-Based Vaccine Induces High Mucosal and Systemic Protecting Immunity Against a SARS-CoV-2 Infection.Front Immunol. 2021 Dec 17;12:781280. doi: 10.3389/fimmu.2021.781280. eCollection 2021. Front Immunol. 2021. PMID: 34987509 Free PMC article.
-
Evaluation of combined BCG and SARS-CoV-2 vaccination for immune enhancement and lung protection in Syrian hamsters.Sci Rep. 2025 May 29;15(1):18908. doi: 10.1038/s41598-025-04246-3. Sci Rep. 2025. PMID: 40442229 Free PMC article.
-
Preclinical immunogenicity and efficacy of a candidate COVID-19 vaccine based on a vesicular stomatitis virus-SARS-CoV-2 chimera.EBioMedicine. 2022 Aug;82:104203. doi: 10.1016/j.ebiom.2022.104203. Epub 2022 Jul 30. EBioMedicine. 2022. PMID: 35915046 Free PMC article.
Cited by
-
Recent advances in the exploration and discovery of SARS-CoV-2 inhibitory peptides from edible animal proteins.Front Nutr. 2024 Feb 7;11:1346510. doi: 10.3389/fnut.2024.1346510. eCollection 2024. Front Nutr. 2024. PMID: 38389797 Free PMC article. Review.
-
Immunogenicity and efficacy of XBB.1.5 rS vaccine against the EG.5.1 variant of SARS-CoV-2 in Syrian hamsters.J Virol. 2024 Oct 22;98(10):e0052824. doi: 10.1128/jvi.00528-24. Epub 2024 Sep 4. J Virol. 2024. PMID: 39230305 Free PMC article.
References
-
- World Health Organization . Who Coronavirus (Covid-19) Dashboard. (2022). Available from: https://covid19.who.int/.
-
- European Medicines Agency . Adapted Vaccine Targeting Ba.4 and Ba.5 Omicron Variants Original Sars-Cov-2 Recommended for Approval. (2022). Available from: https://www.ema.europa.eu/en/news/adapted-vaccine-targeting-ba4-ba5-omic....
-
- European Medicines Agency . Comirnaty. (2023). Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/comirnaty.
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

