Similarities and differences of chemical compositions and physical and functional properties of adjuvant system 01 and army liposome formulation with QS21
- PMID: 36761767
- PMCID: PMC9905621
- DOI: 10.3389/fimmu.2023.1102524
Similarities and differences of chemical compositions and physical and functional properties of adjuvant system 01 and army liposome formulation with QS21
Abstract
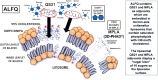
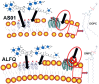
A vaccine adjuvant known as Adjuvant System 01 (AS01) consists of liposomes containing a mixture of natural congeners of monophosphoryl lipid A (MPL®) obtained from bacterial lipopolysaccharide, and a tree saponin known as QS21. Two vaccines containing AS01 as the adjuvant have been licensed, including a malaria vaccine (Mosquirix®) approved by World Health. Organization and European Medicines Agency for use in sub-Saharan Africa, and a shingles vaccine (Shingrix®) approved by the U.S. Food and Drug Administration. The success of the AS01 vaccine adjuvant has led to the development of another liposomal vaccine adjuvant, referred to as Army Liposome Formulation with QS21 (ALFQ). Like AS01, ALFQ consists of liposomes containing monophosphoryl lipid A (as a synthetic molecule known as 3D-PHAD®) and QS21 as adjuvant constituents, and the polar headgroups of the liposomes of AS01 and ALFQ are similar. We compare here AS01 with ALFQ with respect to their similar and different liposomal chemical structures and physical characteristics with a goal of projecting some of the likely mechanisms of safety, side effects, and mechanisms of adjuvanticity. We hypothesize that some of the side effects exhibited in humans after injection of liposome-based vaccines might be caused by free fatty acid and lysophospholipid released by enzymatic attack of liposomal phospholipid by phospholipase A2 at the injection site or systemically after injection.
Keywords: ALFQ; AS01; QS21 (QS-21) saponin; liposomes; monophosphoryl lipid A; phospholipase A 2; vaccine adjuvant.
Copyright © 2023 Alving, Rao and Matyas.
Conflict of interest statement
CA is an inventor on U.S. and International patents for ALFQ. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures
Similar articles
-
Differential immune responses to HIV-1 envelope protein induced by liposomal adjuvant formulations containing monophosphoryl lipid A with or without QS21.Vaccine. 2015 Oct 13;33(42):5578-5587. doi: 10.1016/j.vaccine.2015.09.001. Epub 2015 Sep 13. Vaccine. 2015. PMID: 26372857
-
Army Liposome Formulation (ALF) family of vaccine adjuvants.Expert Rev Vaccines. 2020 Mar;19(3):279-292. doi: 10.1080/14760584.2020.1745636. Epub 2020 Mar 31. Expert Rev Vaccines. 2020. PMID: 32228108 Free PMC article. Review.
-
Safety, toxicity and immunogenicity of a malaria vaccine based on the circumsporozoite protein (FMP013) with the adjuvant army liposome formulation containing QS21 (ALFQ).Vaccine. 2019 Jun 27;37(29):3793-3803. doi: 10.1016/j.vaccine.2019.05.059. Epub 2019 May 28. Vaccine. 2019. PMID: 31151801
-
Saturated phospholipids are required for nano- to micron-size transformation of cholesterol-containing liposomes upon QS21 addition.J Liposome Res. 2019 Sep;29(3):247-250. doi: 10.1080/08982104.2018.1538239. Epub 2018 Nov 23. J Liposome Res. 2019. PMID: 30350748
-
Adjuvant system AS01: from mode of action to effective vaccines.Expert Rev Vaccines. 2024 Jan-Dec;23(1):715-729. doi: 10.1080/14760584.2024.2382725. Epub 2024 Aug 5. Expert Rev Vaccines. 2024. PMID: 39042099 Review.
Cited by
-
Development of a New Vaccine Adjuvant System Based on the Combination of the Synthetic TLR4 Agonist FP20 and a Synthetic QS-21 Variant.J Med Chem. 2024 Dec 26;67(24):22254-22262. doi: 10.1021/acs.jmedchem.4c02392. Epub 2024 Dec 8. J Med Chem. 2024. PMID: 39645607 Free PMC article.
-
Post-Marketing Surveillance of Adverse Events for the Recombinant Zoster Vaccine Among the Population over 50 Years Old in Hangzhou, China.Vaccines (Basel). 2024 Dec 6;12(12):1376. doi: 10.3390/vaccines12121376. Vaccines (Basel). 2024. PMID: 39772038 Free PMC article.
-
Development of semisynthetic saponin immunostimulants.Med Chem Res. 2024;33(8):1292-1306. doi: 10.1007/s00044-024-03227-x. Epub 2024 May 18. Med Chem Res. 2024. PMID: 39132259 Free PMC article. Review.
-
Evolution of Vaccines Formulation to Tackle the Challenge of Anti-Microbial Resistant Pathogens.Int J Mol Sci. 2023 Jul 27;24(15):12054. doi: 10.3390/ijms241512054. Int J Mol Sci. 2023. PMID: 37569427 Free PMC article. Review.
-
Unconjugated Multi-Epitope Peptides Adjuvanted with ALFQ Induce Durable and Broadly Reactive Antibodies to Human and Avian Influenza Viruses.Vaccines (Basel). 2023 Sep 8;11(9):1468. doi: 10.3390/vaccines11091468. Vaccines (Basel). 2023. PMID: 37766144 Free PMC article.
References
-
- Alving CR. Liposomes as carriers for vaccines. In: Ostro M, editor. Liposomes: From biophysics to therapeutics. New York: Marcel Dekker; (1987). p. 195–218.
-
- Garcon NM, Friede M. Vaccines containing a saponin and a sterol. international application number: PCT/EP96/01464. World Intellectual Property Organization International Publication Number WO 96/33739; (1996). Available at: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO1996033739.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

